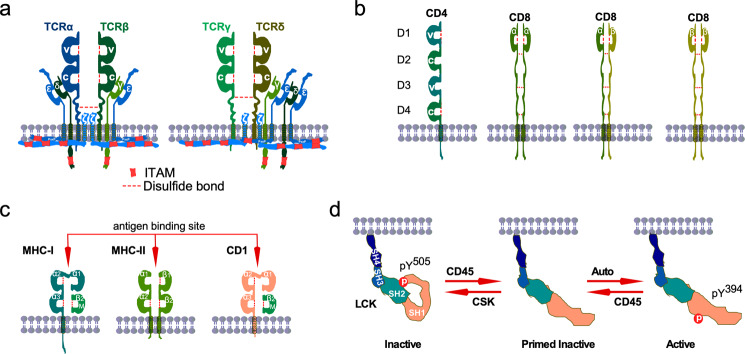
Fig. 1. TCR components.
a TCRα/TCRβ and TCRγ/TCRδ heterodimers form complexes with the CD3 molecules. Heterodimers of CD3ε/CD3δ and CD3γ/CD3ε, and a homodimer of CD3ζ/CD3ζ form complexes with TCR dimers. TCR heterodimers contain intramolecular and intermolecular disulfide bonds. CD3 chains contain 10 ITAMs distributed in different CD3 molecules. The variable region (V) of TCR heterodimers recognize the antigen peptide-loaded on MHC (pMHC). In the absence of pMHC, the intracellular part of the CD3 molecules forms a close conformation in which ITAMs are inaccessible to the kinases for phosphorylation. b Coreceptor CD4 acts as a single molecule while CD8α and CD8β can form homodimers or heterodimers. c MCH-I consists of an α-chain containing three immunoglobulin domains (α1, α2, α3) and β2-microglobulin (β2m). MCH-2 is the heterodimer of an α chain and a β-chain containing two immunoglobulin domains (α1, α2, and β1, β2) in each chain. d LCK-loaded CD4 molecules bind to the MHC-II bound TCR (TCRα/TCRβ) complex. This allows LCK to phosphorylate two distinct sites on ITAMs. Then ZAP-70 interacts with the phosphotyrosine sites and mediates more tyrosine phosphorylation. CD4 and MHC-II interaction is mediated through the membrane-proximal α2 and β2 domains of MHC-II and the membrane-distal D1 domain of CD4.