Abstract
Purpose: We sought to enumerate secondary medical conditions from hospitalization records in adolescent and young adult (AYA) differentiated thyroid cancer (DTC) survivors and identify characteristics of patients with increased likelihood of subsequent medical diagnoses.
Methods: Using data from the California Cancer Registry and statewide hospitalization data, we examined incident oncologic, endocrine, pulmonary, hematologic, and cardiovascular diagnoses in 12,312 AYA (aged 15–39) patients diagnosed with DTC in 1996–2012 and surviving >2 years after diagnosis with follow-up through 2014. We calculated the cumulative incidence of each condition accounting for the competing risk of death and used multivariable Cox proportional hazards regression to evaluate sociodemographic and clinical characteristics associated with each incident condition.
Results: The 10-year cumulative incidences of multiple medical conditions were particularly high in blacks and Hispanics. Asian/Pacific Islander survivors were most likely to develop subsequent cancers. Men had higher rates of cardiovascular and diabetes diagnoses than women, but lower rates of asthma and cytopenias. Low socioeconomic status and/or public or no insurance were associated with a higher risk of several diagnoses. More extensive disease stage and thyroid surgery increased the risk of calcium and phosphorus metabolism disorders. Neck reoperation associated with the risk of cytopenias, as well as subsequent endocrine, cardiovascular, and respiratory diagnoses.
Conclusion: The incidence of medical conditions after thyroid cancer diagnosis and treatment differ among racial/ethnic groups and sexes. Those residing in lower socioeconomic neighborhoods, those with public or no insurance, and those who require further neck surgery have substantially higher burdens of subsequent medical diagnoses.
Keywords: thyroid cancer, late effects, cancer survivorship, socioeconomic factors
Introduction
Since about 2000, the number of differentiated thyroid cancers (DTCs) diagnosed across the world has risen dramatically in adult and pediatric populations.1–5 This thyroid cancer epidemic is a result, at least partially, of the diagnosis of a significant number of lower risk papillary thyroid carcinomas.3 Nonetheless, within this worldwide tidal wave of thyroid cancer diagnoses, the number of larger and higher risk DTCs has also been on the rise.2 Moreover, the mortality rates from thyroid cancer have been rising on average 0.7% each year from 2006 to 2015.6 Thus, significant effort is being expended to appropriately target interventions that reduce the morbidity and mortality associated with the diagnosis and treatment of DTC as well as to avoid overtreatment of a lower risk DTC patient population.
Due to the young average age of diagnosis of DTC, the adolescent and young adult (AYA) population diagnosed between 15 and 39 years of age is disproportionately impacted by the thyroid cancer epidemic. The primary initial treatment of DTC is surgical removal of the thyroid gland.7 Known consequences of surgical thyroid removal include laryngeal nerve injury and hypoparathyroidism along with hypothyroidism necessitating thyroid hormone replacement.8–10 Depending on recurrence risk, surgery is followed by treatment with radioiodine in ∼54.5% of the AYA population,11 and differing degrees of thyroid stimulating hormone (TSH) suppression. Radioiodine may lead to salivary gland dysfunction and pulmonary fibrosis while TSH suppression has been associated with an increased risk of cardiovascular disease.12–15 Thyroid cancer survivors experience higher risks of second cancers, aging-related diseases, and hospitalizations than AYAs without cancer.16–20
We previously documented that the initial treatment of AYA diagnosed with DTC is more intense than in older adults, with an increased use of complete thyroid surgery and increased rates of initial treatment with radioiodine,11 potentially resulting in higher risks of adverse medical conditions among AYAs than older adults diagnosed with DTC.19 Moreover, we demonstrated worse survival outcomes after diagnoses of DTC in certain AYA sociodemographic groups, namely men, including those residing in low socioeconomic status (SES) neighborhoods, and AYAs of African American or Hispanic race/ethnicity.21 Young men and those with public or no health insurance were more likely to die from other, noncancer causes of death,21 but no study to our knowledge has considered whether these sociodemographic factors are associated with subsequent medical conditions in AYA DTC survivors.
Thyroid cancer survivors may develop subsequent medical conditions that are direct consequences of thyroid cancer treatment, disorders that are plausibly linked to the disease or its treatment, and/or experience subsequent diagnoses that are not specifically related to thyroid cancer diagnosis or its treatment. Strategies to improve survivorship among cancer survivors need to address medical problems that develop by all of these mechanisms. Given the rapidly increasing incidence of DTC and its disproportionate impact on the AYA population, we sought to identify secondary medical conditions from hospitalization records in AYA thyroid cancer survivors and understand sociodemographic and clinical characteristics of patients with increased likelihood of these serious late effects. The results of this inquiry could have implications on shared decision-making on the initial treatment of DTC in AYA populations and for medical surveillance after initial treatment is complete.
Materials and Methods
Setting and subjects
Eligible subjects were diagnosed with a first primary DTC between 15 and 39 years of age in California between January 1, 1996 and December 31, 2012. Subjects were identified from the California Cancer Registry (CCR) using the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), histology codes 8050, 8260, and 8340–8344 to define papillary thyroid cancers and histology codes 8290, 8330–8332, and 8335 to define follicular cancers. The CCR is the largest population-based cancer registry in the United States, with records from the cancer diagnosis of all state residents diagnosed with cancer except those from military or Veterans Administration facilities. Using an encrypted form of the social security number and gender, we linked new DTC cases to hospital discharges from the State of California Office of Statewide Health Planning and Development (OSHPD). This database contains detailed diagnosis and procedure data from each discharge from any non-Federal hospital in California.
Tumor, sociodemographic, and treatment variables
From the CCR, we obtained information from the time of diagnosis, including age, sex, race/ethnicity, summary stage, and census-block group of residence. We obtained neighborhood SES from the CCR, which is a multicomponent index of United States. Census characteristics (education, occupation, unemployment, household income, poverty, rent, and house values) based on residential census-block group at diagnosis.22 The index is grouped into quintiles, which we grouped into two categories—lower SES (quintiles 1–3) and higher SES (quintiles 4, 5). We categorized insurance categories of public (MediCal and other government-assisted programs), private/military (health maintenance organizations, preferred provider organizations, and managed care not otherwise specified), none (self-pay), and unknown from CCR information on the primary source of payment at diagnosis. Follow-up time and vital status was obtained through December 2014.
Extent of thyroid surgery was defined using International Classification of Diseases, 9th Revision, Clinical Modifications (ICD-9-CM) for total or near total thyroidectomy in OSHPD or most extensive surgery at initial treatment from CCR. Patients with two lobectomies within 90 days in OSHPD were classified as having total or near total thyroidectomy. The date of thyroid surgery was identified as the OSHPD procedure date or the recorded CCR date. Registry data on the administration of radioiodine as part of initial treatment were obtained from the CCR. Due to the potential for increased morbidity and mortality in patients requiring subsequent neck surgery, we defined neck reoperation from OSHPD as a neck lymph node surgery 91 days to 5 years after the initial thyroid surgery as previously reported.23 Tobacco use disorder and overweight/obesity were captured from OSPHD up to 2 years before the DTC diagnosis.
Incident medical conditions
From OSHPD, we obtained information for hospitalizations that occurred at least 2 years after diagnosis, including the principal diagnosis and up to 24 other diagnoses, as well as up to 20 procedures and their corresponding dates for hospitalizations. These records are coded using the ICD-9-CM. To separate incidental medical conditions by clinically relevant categories, we grouped relevant hospital discharge diagnoses present ≥2 years after diagnosis into five systems that included oncologic, endocrine, pulmonary, hematologic, and cardiovascular diagnoses. Second cancers were identified by the CCR. We further categorized endocrine diagnoses as disorders of calcium and phosphorus metabolism or diabetes mellitus, as the former can be causally attributed to parathyroid injury during initial thyroid cancer treatment. We divided pulmonary diagnoses into asthma and other specified infections/inflammatory respiratory disease, the latter including pulmonary fibrosis that may be the direct result of radioiodine treatment and aspiration that may plausibly be linked to subsequent salivary and swallowing disorders. We grouped hematologic effects into cytopenias (leukopenia, anemia, and/or thrombocytopenia) and leukocytosis as opposites, and separated hypertension from other disease of the heart based on prior studies that have specifically linked these disorders to DTC diagnosis and treatment (Table 1).19,24 To focus on medical conditions emerging after thyroid cancer diagnosis and treatment, we excluded medical conditions present before DTC diagnosis as outcomes.
Table 1.
Incident Medical Condition Codes Using International Classification of Diseases, Ninth Edition Nomenclature
| Class | ICD-9 code | Name | Count |
|---|---|---|---|
| Endocrine—Disorders of calcium and phosphorus metabolism | |||
| 252.1 | Hypoparathyroidism | 101 | |
| 275.41 | Hypocalcemia | 135 | |
| 275.42 | Hypercalcemia | 21 | |
| 275.3 | Disorders of phosphorus metabolism | 39 | |
| Endocrine—Diabetes | |||
| 250 | Diabetes mellitus | 245 | |
| Respiratory—Asthma | |||
| 493 | Asthma | 272 | |
| Respiratory—Other infectious/inflammatory respiratory diseases | |||
| 460–466 | Acute upper respiratory infections | 61 | |
| 480–488 | Pneumonia and influenza | 117 | |
| 490–492 | Bronchitis and emphysema | 33 | |
| 507 | Pneumonitis due to solids and liquids | 35 | |
| 515 | Postinflammatory pulmonary fibrosis | 8 | |
| Hematologic—Cytopenias | |||
| 288.0, 288.5 | Hematologic cytopenia (neutropenia + decreased white blood cells) | 37 | |
| 280–285 | Anemia | 686 | |
| 287.3–287.5 | Thrombocytopenia | 85 | |
| Hematologic—Leukocytosis | |||
| 288.3 | Eosinophilia | 3 | |
| 288.6 | Elevated white blood cell count | 81 | |
| 288.8 | Other specified disease of white blood cells | 9 | |
| Cardiovascular—Hypertension | |||
| 401–405 | Hypertension | 562 | |
| Cardiovascular—Diseases of the heart | |||
| 393–398 | Chronic rheumatic heart disease | 13 | |
| 410–414 | Ischemic heart disease | 71 | |
| 420–429 | Other forms of heart disease | 237 | |
ICD-9, International Classification of Diseases, Ninth Edition.
Statistical analyses
The cumulative incidence and associated 95% confidence intervals (CIs) of developing a medical condition ≥2 years after diagnosis was calculated using nonparametric methods that account for death as a competing risk.25 Person-years of observation were compiled from 2 years after DTC diagnosis to date of first hospitalization with a specified medical condition (multiple incident medical conditions were possible), the date of last known contact, date of death, or the study cutoff date (December 31, 2014), whichever occurred first. Gray's K-sample test statistic was used to determine whether cumulative incidence of a medical condition differed by sociodemographic or clinical factors.26
To evaluate sociodemographic and clinical characteristics associated with the occurrence of each medical condition ≥2 years after diagnosis, we used multivariable Cox proportional hazards regression to calculate adjusted hazard ratios and 95% CIs. Event time was measured in days from 2 years after diagnosis to the date of first identification of each medical condition. Patients without a medical condition at the study end date (December 31, 2014) were censored at this date or at the date of last known contact; deaths from any cause were considered as competing risks in these analyses. The regression models included age at cancer diagnosis, stage at diagnosis, histology, sex, race/ethnicity, year of diagnosis, health insurance status, neighborhood SES, overweight/obesity, tobacco use disorder, and treatment. In all models, the proportional hazards assumption was assessed numerically based on cumulative sums of Martingale residuals and visually based on inspection of the survival curves [log (−log) of the survival distribution function by log (months)]; no variables violated this assumption. Multicollinearity was assessed by examining variance inflation factors (VIF). All models met our criteria of nonmulticollinearity with VIF <10. Analyses were conducted using SAS version 9.4 software (SAS Institute, Inc., Cary, NC). All study protocols were overseen by the Institutional Review Board of the University of California, Davis and by the California Committee for the Protection of Human Subjects.
Results
Study population
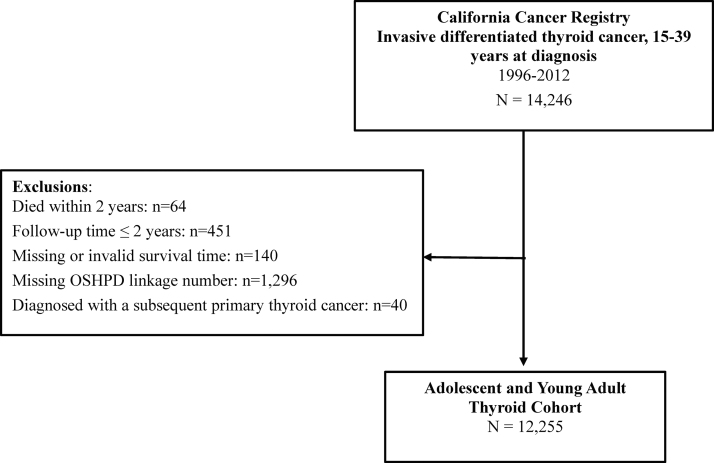
A total of 14,246 DTC cases arising in AYA were identified from the CCR from 1996 to 2012. After exclusion of those who died within 2 years (n = 64), those with <2 years of follow-up (n = 451), those with an unknown/invalid linkage to hospitalization data (n = 1296), those with missing or invalid survival time (n = 140), and those with a subsequent, second primary thyroid cancer (n = 40), a total of 12,255 AYAs were included in these analyses (Fig. 1).
FIG. 1.
Derivation of analysis cohort of adolescent and young adult differentiated thyroid cancer patients, California, 1996–2012.
The majority of AYA DTC survivors in California were women (83.8%) and diagnosed with localized/regional disease (96.0%). The study population was 50.0% non-Hispanic white, 30.5% Hispanic, 15.3% Asian, and 2.9% black (Table 2). Hispanics were more frequently younger than other racial/ethnic groups and even less likely to be male. Follicular carcinoma was more common in black AYA DTC survivors than among other racial/ethnic groups. Non-Hispanic whites and Asian/Pacific Islanders were more likely than black or Hispanics to have private health insurance and reside in high SES neighborhoods. The most common initial treatment included total thyroidectomy followed by radioiodine (in 51.1% overall), with blacks more likely to undergo partial or no thyroidectomy (20.7%) than AYAs of other race/ethnicities (compared to 17.3% of the non-Hispanic white, 17.4% of the Hispanic, and 15.8% of the Asian populations).
Table 2.
Selected Characteristics of Two-Year Survivors of Adolescent and Young Adult Differentiated Thyroid Cancer by Race/Ethnicity, California, 1996–2012
| |
Total |
Non-Hispanic White |
Black |
Hispanic |
Asian/Pacific Islander |
p Value |
|---|---|---|---|---|---|---|
| Baseline characteristic [No. (%)] | (n = 12,255) | (n = 6122) | (n = 357) | (n = 3735) | (n = 1870) | |
| Age at diagnosis | ||||||
| 15–29 | 4597 (37.5) | 2211 (36.1) | 134 (37.5) | 1563 (41.8) | 623 (33.3) | <0.01 |
| 30–34 | 3420 (27.9) | 1713 (28.0) | 98 (27.5) | 1031 (27.6) | 532 (28.4) | |
| 35–39 | 4238 (34.6) | 2198 (35.9) | 125 (35.0) | 1141 (30.5) | 715 (38.2) | |
| Sex | ||||||
| Female | 10,269 (83.8) | 5019 (82.0) | 304 (85.2) | 3229 (86.5) | 1563 (83.6) | <0.01 |
| Male | 1986 (16.2) | 1103 (18.0) | 53 (14.8) | 506 (13.5) | 307 (16.4) | |
| Year of diagnosis | ||||||
| 1996–2000 | 2881 (23.5) | 1608 (26.3) | 81 (22.7) | 793 (21.2) | 367 (19.6) | <0.01 |
| 2001–2004 | 2676 (21.8) | 1396 (22.8) | 83 (23.2) | 759 (20.3) | 408 (21.8) | |
| 2005–2008 | 3101 (25.3) | 1495 (24.4) | 85 (23.8) | 957 (25.6) | 511 (27.3) | |
| 2009–2012 | 3597 (29.4) | 1623 (26.5) | 108 (30.3) | 1226 (32.8) | 584 (31.2) | |
| Histology | ||||||
| Follicular | 920 (7.5) | 478 (7.8) | 53 (14.8) | 236 (6.3) | 133 (7.1) | <0.01 |
| Papillary | 11,335 (92.5) | 5644 (92.2) | 304 (85.2) | 3499 (93.7) | 1737 (92.9) | |
| Stage at diagnosis | ||||||
| Localized/regional | 11,769 (96.0) | 5915 (96.6) | 343 (96.1) | 3559 (95.3) | 1792 (95.8) | <0.01 |
| Advanced | 365 (3.0) | 145 (2.4) | 10 (2.8) | 144 (3.9) | 62 (3.3) | |
| Missing | 121 (1.0) | 62 (1.0) | 4 (1.1) | 32 (0.9) | 16 (0.9) | |
| Neighborhood socioeconomic status | ||||||
| Low | 6417 (52.4) | 2503 (40.9) | 256 (71.7) | 2782 (74.5) | 777 (41.6) | <0.01 |
| High | 5838 (47.6) | 3619 (59.1) | 101 (28.3) | 953 (25.5) | 1093 (58.4) | |
| Health insurance | ||||||
| Private/military | 10,444 (85.2) | 5489 (89.7) | 274 (76.8) | 2892 (77.4) | 1658 (88.7) | <0.01 |
| Public/none | 1627 (13.3) | 568 (9.3) | 77 (21.6) | 772 (20.7) | 185 (9.9) | |
| Unknown | 184 (1.5) | 65 (1.1) | 6 (1.7) | 71 (1.9) | 27 (1.4) | |
| Initial treatment | ||||||
| Total thyroidectomy and radioiodine | 6262 (51.1) | 3120 (51.0) | 156 (43.7) | 1954 (52.3) | 955 (51.1) | 0.03 |
| Total thyroidectomy without radioiodine | 3883 (31.7) | 1944 (31.8) | 127 (35.6) | 1132 (30.3) | 619 (33.1) | |
| Partial/no thyroidectomy | 2110 (17.2) | 1058 (17.3) | 74 (20.7) | 649 (17.4) | 296 (15.8) | |
| Neck reoperation | ||||||
| Yes | 754 (6.2) | 357 (5.8) | 17 (4.8) | 249 (6.7) | 125 (6.7) | 0.14 |
| No | 11,501 (93.8) | 5765 (94.2) | 340 (95.2) | 3486 (93.3) | 1745 (93.3) | |
| Tobacco use | ||||||
| Yes | 252 (2.1) | 151 (2.5) | 10 (2.8) | 77 (2.1) | 10 (0.5) | <0.01 |
| No | 12,003 (97.9) | 5971 (97.5) | 347 (97.2) | 3658 (98.0) | 1860 (99.5) | |
| Obesity | ||||||
| Yes | 430 (3.5) | 176 (2.9) | 25 (7.0) | 198 (5.3) | 25 (1.3) | <0.01 |
| No | 11,825 (96.5) | 5946 (97.1) | 332 (93.0) | 3537 (94.7) | 1845 (98.7) | |
Cumulative incidence
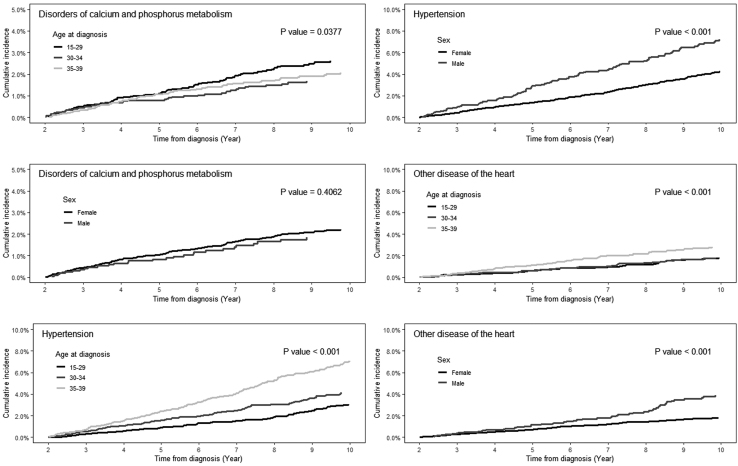
The median follow-up was 8.8 years (Interquartile range 5.13–13.24 years). Male DTC survivors had a higher 10-year cumulative incidence of diabetes mellitus and cardiovascular disease, but lower cumulative incidence of asthma and hematologic disorders (Table 3). Within this AYA population, the cumulative incidence of subsequent cancers, diabetes mellitus, leukocytosis, and cardiovascular diagnoses increased with age at diagnosis. For disorders of calcium and phosphorus metabolism, hypertension, and other diseases of the heart—disorders that prior studies have identified as consequences of the diagnosis and treatment of DTC—we observed that the incidence rose continuously between 2 and 10 years from diagnosis, but at different rates based on age and sex (Table 3; Fig. 2). Black DTC survivors had the highest 10-year cumulative incidence of diabetes mellitus, asthma, other infections/inflammatory respiratory disorders, cytopenias, hypertension, and other disease of the heart, but the lowest incidence of subsequent cancers. Hispanics had the highest incidence of disorders of calcium and phosphorus metabolism and a higher incidence of diabetes and cytopenias than non-Hispanic whites and Asian/Pacific Islanders.
Table 3.
Cumulative Incidence and Associated 95% Confidence Intervals of Incident Medical Conditions Ten Years After an Initial Diagnosis Among Two-Year Adolescent And Young Adult Thyroid Survivors, California, 1996–2012
| Baseline characteristic [cumulative incidence (95% CI)] | Oncologic |
Endocrine |
Respiratory |
Hematologic |
Cardiovascular |
||||
|---|---|---|---|---|---|---|---|---|---|
| Subsequent cancers | Disorders of calcium and phosphorus metabolism | Diabetes mellitus | Asthma | Other infectious/inflammatory respiratory diseases | Cytopenias | Leukocytosis | Hypertension | Other diseases of the heart | |
| Sex | |||||||||
| Female | 2.11 (1.78–2.48) | 2.22 (1.90–2.58) | 1.81 (1.52–2.14) | 2.45 (2.11–2.83) | 1.41 (1.15–1.71) | 6.61 (6.04–7.22) | 0.49 (0.35–0.67) | 4.25 (3.75–4.76) | 1.81 (1.52–2.14) |
| Male | 2.21 (1.51–3.12) | 1.84 (1.24–2.63) | 3.91 (2.94–5.09) | 1.38 (0.85–2.15) | 2.23 (1.55–3.10) | 3.67 (2.75–4.79) | 0.82 (0.44–1.41) | 7.24 (5.92–8.72) | 3.91 (2.94–5.09) |
| p Value | 0.74 | 0.41 | <0.01 | <0.01 | 0.01 | <0.01 | 0.16 | <0.01 | <0.01 |
| Age at diagnosis | |||||||||
| 15–29 | 1.33 (0.97–1.79) | 2.61 (2.10–3.20) | 1.35 (0.99–1.81) | 2.39 (1.89–2.98) | 1.50 (1.12–1.97) | 6.52 (5.68–7.43) | 0.38 (0.21–0.65) | 3.03 (2.44–3.71) | 1.82 (1.38–2.35) |
| 30–34 | 2.34 (1.75–3.06) | 1.67 (1.22–2.23) | 2.01 (1.49–2.65) | 2.19 (1.66–2.84) | 1.66 (1.19–2.27) | 6.01 (5.08–7.04) | 0.73 (0.46–1.11) | 4.13 (3.37–5.01) | 1.81 (1.32–2.42) |
| 35–39 | 2.81 (2.24–3.48) | 2.06 (1.60–2.62) | 3.01 (2.43–3.69) | 2.22 (1.74–2.80) | 1.50 (1.11–1.98) | 5.81 (4.98–6.71) | 0.57 (0.35–0.91) | 7.08 (6.15–8.08) | 2.8 (2.25–3.45) |
| p Value | <0.01 | 0.04 | <0.01 | 0.75 | 0.37 | 0.44 | <0.01 | <0.01 | <0.01 |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 2.11 (1.70–2.59) | 1.86 (1.50–2.28) | 1.47 (1.14–1.86) | 2.40 (1.98–2.88) | 1.60 (1.25–2.01) | 5.09 (4.46–5.78) | 0.47 (0.30–0.71) | 4.11 (3.53–4.74) | 1.96 (1.57–2.41) |
| Black | 0.76 (0.15–2.53) | 1.63 (0.53–3.94) | 4.02 (2.07–6.95) | 4.45 (2.31–7.67) | 2.85 (1.31–5.38) | 9.44 (6.15–13.56) | 0.67 (0.14–2.25) | 11.27 (7.67–15.64) | 4.27 (2.31–7.15) |
| Hispanic | 2.05 (1.53–2.69) | 3.03 (2.41–3.76) | 3.24 (2.57–4.01) | 2.68 (2.08–3.39) | 1.55 (1.11–2.11) | 7.48 (6.45–8.61) | 0.69 (0.42–1.07) | 5.68 (4.77–6.70) | 2.28 (1.73–2.95) |
| Asian/Pacific Islander | 2.61 (1.80–3.64) | 1.56 (0.98–2.36) | 1.71 (1.08–2.58) | 0.77 (0.43–1.30) | 0.93 (0.50–1.60) | 6.27 (4.97–7.76) | 0.54 (0.24–1.09) | 3.70 (2.75–4.86) | 2.19 (1.46–3.16) |
| p Value | 0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.30 | <0.01 | 0.06 |
| Year of diagnosis | |||||||||
| 1996–2000 | 2.18 (1.69–2.77) | 1.36 (0.99–1.84) | 2.00 (1.53–2.57) | 1.89 (1.44–2.45) | 1.33 (0.96–1.80) | 5.69 (4.88–6.58) | 0.25 (0.11–0.49) | 4.96 (4.20–5.80) | 2.28 (1.78–2.88) |
| 2001–2004 | 1.63 (1.20–2.17) | 2.16 (1.66–2.77) | 2.13 (1.63–2.73) | 2.28 (1.76–2.90) | 1.97 (1.49–2.56) | 5.69 (4.85–6.61) | 0.53 (0.31–0.87) | 4.44 (3.70–5.28) | 2.01 (1.53–2.60) |
| 2005–2008a | |||||||||
| 2009–2012a | |||||||||
| p Value | 0.18 | <0.01 | 0.91 | 0.23 | 0.53 | 0.10 | 0.02 | 0.40 | 0.51 |
| Histology | |||||||||
| Follicular | 1.14 (0.53–2.18) | 1.85 (1.00–3.15) | 1.92 (1.06–3.21) | 2.77 (1.71–4.24) | 1.12 (0.52–2.16) | 6.21 (4.56–8.18) | 0.28 (0.06–0.99) | 5.13 (3.60–7.03) | 2.10 (1.19–3.44) |
| Papillary | 2.22 (1.89–2.58) | 2.18 (1.88–2.52) | 2.13 (1.82–2.47) | 2.23 (1.92–2.58) | 1.58 (1.32–1.88) | 6.13 (5.60–6.69) | 0.57 (0.42–0.75) | 4.70 (4.23–5.21) | 2.16 (1.85–2.51) |
| p Value | 0.81 | 0.43 | 0.68 | 0.33 | 0.77 | 0.84 | 0.34 | 0.36 | 0.92 |
| Stage at diagnosis | |||||||||
| Localized/regional | 2.09 (1.79–2.44) | 2.04 (1.75–2.36) | 2.05 (1.75–2.39) | 2.35 (2.03–2.70) | 1.45 (1.21–1.74) | 6.05 (5.53–6.60) | 0.50 (0.37–0.67) | 4.68 (4.22–5.17) | 2.12 (1.82–2.46) |
| Advanced | 2.62 (1.15–5.13) | 4.79 (2.77–7.61) | 3.70 (1.94–6.34) | b | 4.38 (2.36–7.32) | 9.15 (6.16–12.85) | 1.52 (0.50–3.66) | 6.31 (3.80–9.70) | 2.09 (0.86–4.32) |
| p Value | 0.93 | <0.01 | 0.56 | 0.62 | 0.02 | 0.11 | 0.72 | 0.80 | 0.42 |
| Neighborhood SES | |||||||||
| Low | 1.92 (1.53–2.37) | 2.64 (2.20–3.14) | 2.71 (2.25–3.24) | 1.93 (1.54–2.37) | 2.85 (2.38–3.38) | 7.02 (6.27–7.82) | 0.70 (0.49–0.98) | 5.28 (4.63–5.99) | 2.51 (2.07–3.02) |
| High | 2.35 (1.90–2.88) | 1.64 (1.30–2.05) | 1.46 (1.13–1.87) | 1.14 (0.84–1.50) | 1.66 (1.31–2.08) | 5.18 (4.52–5.91) | 0.38 (0.23–0.60) | 4.15 (3.54–4.83) | 1.78 (1.41–2.23) |
| p Value | 0.20 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.02 |
| Health insurance | |||||||||
| Private/military | 2.20 (1.87–2.57) | 1.85 (1.56–2.17) | 1.71 (1.43–2.03) | 1.18 (0.95–1.45) | 2.04 (1.73–2.38) | 5.69 (5.16–6.25) | 0.40 (0.28–0.56) | 4.12 (3.67–4.61) | 1.81 (1.52–2.14) |
| Public/none | 1.63 (0.97–2.57) | 4.41 (3.28–5.79) | 4.21 (3.08–5.58) | 3.87 (2.80–5.19) | 3.97 (2.91–5.29) | 9.03 (7.37–10.89) | 1.41 (0.84–2.24) | 8.50 (6.84–10.39) | 4.69 (3.47–6.17) |
| p Value | 0.50 | <0.01 | <0.001 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Initial treatment | |||||||||
| Total thyroidectomy and radioiodine | 2.41 (1.97–2.91) | 2.16 (1.76–2.61) | 1.80 (1.43–2.23) | 2.17 (1.77–2.64) | 1.54 (1.20–1.95) | 5.98 (5.29–6.74) | 0.33 (0.19–0.53) | 4.56 (3.95–5.24) | 2.16 (1.75–2.64) |
| Total thyroidectomy without radioiodine | 1.75 (1.27–2.36) | 2.63 (2.07–3.30) | 2.41 (1.85–3.08) | 2.66 (2.07–3.36) | 1.43 (1.01–1.96) | 7.15 (6.17–8.22) | 0.71 (0.44–1.08) | 5.02 (4.18–5.97) | 2.05 (1.54–2.67) |
| Partial/no thyroidectomy | 1.94 (1.33–2.74) | 1.39 (0.91–2.07) | 2.43 (1.74–3.30) | 1.99 (1.40–2.76) | 1.78 (1.22–2.51) | 4.94 (3.95–6.09) | 0.86 (0.49–1.41) | 4.76 (3.78–5.90) | 2.31 (1.66–3.15) |
| p Value | 0.81 | <0.01 | 0.38 | 0.55 | 0.58 | <0.01 | <0.01 | 0.70 | 0.86 |
| Neck reoperation | |||||||||
| Yes | 2.10 (1.07–3.74) | 4.90 (3.38–6.80) | 3.46 (2.04–5.45) | 3.94 (2.51–5.85) | 2.84 (1.52–4.84) | 6.39 (4.36–8.94) | 0.29 (0.06–0.99) | 7.12 (5.07–9.61) | 2.92 (1.68–4.70) |
| No | 2.13 (1.82–2.48) | 1.98 (1.69–2.30) | 2.03 (1.73–2.36) | 2.17 (1.87–2.51) | 1.47 (1.22–1.76) | 6.12 (5.60–6.67) | 0.56 (0.42–0.74) | 4.58 (4.12–5.08) | 2.11 (1.80, 2.45) |
| p Value | 0.50 | <0.01 | 0.04 | <0.01 | 0.04 | 0.03 | 0.44 | <0.01 | 0.07 |
Incident medical conditions obtained from hospital discharge records.
Insufficient follow-up to obtain 10 years cumulative incidence.
There were too few events to compute incidence.
CI, confidence interval; SES, socioeconomic status.
FIG. 2.
Cumulative incidence of disorders of calcium and phosphorus metabolism, hypertension, and other disease of the heart from 2 to 10 years after diagnosis of differentiated thyroid cancer, by age category and sex.
There was no difference in the 10-year cumulative incidence of late effects by DTC histology. Those with more advanced disease at diagnosis were more likely to develop a disorder of calcium and phosphorus metabolism and an inflammatory/infectious respiratory disease. Incidence was generally higher among those residing in low SES neighborhoods and those with public or no insurance.
More extensive initial surgery was associated with a higher 10-year cumulative incidence of calcium and phosphorus metabolism disorders. The 10-year cumulative incidence of an incident hematologic cytopenia diagnosis was highest in the group treated with total thyroidectomy without radioiodine, while leukocytosis was lowest in those treated with total thyroidectomy followed by radioiodine. Neck reoperation among AYA DTC survivors was associated with a higher 10-year cumulative incidence of multiple diagnoses, including disorders of calcium and phosphorus metabolism, diabetes mellitus, cytopenias, hypertension, and each category of respiratory diagnoses.
Risk of medical conditions
In multivariable models, women were more likely to develop asthma and cytopenias, but less likely to develop diabetes, other respiratory disorders, and cardiovascular late effects (Table 4). The most common incident diagnoses noted in admissions for labor or delivery were cytopenias (in 11.2%) and asthma (in 4.5%). However, we did not observe significant changes in the differences between males and females after excluding outcomes that only arose in a labor/delivery admission in a sensitivity analysis (data not shown). Older AYA survivors were more likely to develop disease associated with aging such as subsequent cancers, diabetes mellitus, and cardiovascular diseases. Asian/Pacific Islander survivors were over 50% more likely to develop subsequent cancers than non-Hispanic whites. The risk of developing calcium and phosphorus metabolism disorders, 80% of which were hypoparathyroidism or hypocalcemia, was increased in AYAs diagnosed between 2005 and 2008, with advanced disease stage, more extensive surgery, and among those undergoing a neck reoperation. Diabetes mellitus developed more commonly among Hispanic, black, and male AYA DTC survivors. Asian/Pacific Islanders were less likely than non-Hispanic whites to develop a respiratory late effect, while blacks were more likely to develop a cardiovascular diagnosis. Public insurance and low neighborhood SES were associated with emergence of a number of subsequent medical conditions. AYAs undergoing a neck reoperation experienced a higher risk of endocrine disorders, respiratory conditions, hematologic cytopenia, and cardiovascular diagnoses.
Table 4.
Multivariable Cox Proportional Hazards Regression Models Assessing the Association of Each Incident Medical Condition with Baseline Demographic and Clinical Characteristics
| Baseline characteristic [HR (95% CI)a] | Oncologic |
Endocrine |
Respiratory |
Hematologic |
Cardiovascular |
||||
|---|---|---|---|---|---|---|---|---|---|
| Subsequent cancers | Disorders of calcium and phosphorus metabolism | Diabetes mellitus | Asthma | Other infectious/inflammatory respiratory diseases | Cytopenias | Leukocytosis | Hypertension | Diseases of the heart | |
| Sex | |||||||||
| Male | Reference | Reference | Reference | Reference | Refence | Reference | Reference | Reference | Reference |
| Female | 1.11 (0.81–1.52) | 1.17 (0.82–1.68) | 0.60 (0.45–0.82) | 2.22 (1.43–3.44) | 0.62 (0.44–0.87) | 1.65 (1.30–2.08) | 0.69 (0.42–1.15) | 0.66 (0.54–0.81) | 0.49 (0.38–0.65) |
| Age at diagnosis | |||||||||
| 15–29 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 30–34 | 1.79 (1.30–2.48) | 0.73 (0.52–1.03) | 1.53 (1.08–2.16) | 0.98 (0.73–1.33) | 1.12 (0.77–1.62) | 0.98 (0.81–1.17) | 2.47 (1.41–4.33) | 1.62 (1.27–2.06) | 1.30 (0.93–1.83) |
| 35–39 | 2.42 (1.79–3.27) | 0.99 (0.74–1.32) | 2.12 (1.55–2.91) | 1.01 (0.76–1.33) | 1.35 (0.96–1.90) | 0.98 (0.82–1.16) | 2.19 (1.27–3.77) | 2.83 (2.29–3.51) | 2.16 (1.61–2.90) |
| Race/ethnicity | |||||||||
| Non-Hispanic White | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Hispanic | 1.06 (0.79–1.42) | 1.33 (0.99–1.79) | 1.65 (1.22–2.24) | 0.91 (0.69–1.22) | 0.83 (0.59–1.18) | 1.28 (1.07–1.52) | 1.06 (0.64–1.74) | 1.25 (1.02–1.53) | 1.06 (0.79–1.43) |
| Asian/Pacific Islander | 1.57 (1.16–2.11) | 0.93 (0.62–1.40) | 0.97 (0.62–1.50) | 0.38 (0.22–0.63) | 0.47 (0.26–0.83) | 1.06 (0.85–1.34) | 0.64 (0.30–1.36) | 1.05 (0.81–1.37) | 1.05 (0.72–1.52) |
| Black | 0.75 (0.34–1.65) | 0.70 (0.28–1.72) | 2.29 (1.30–4.03) | 1.56 (0.92–2.65) | 1.44 (0.74–2.78) | 1.57 (1.08–2.28) | 0.86 (0.26–2.90) | 2.02 (1.37–2.97) | 1.89 (1.09–3.27) |
| Other/unknown | 0.59 (0.14–2.44) | 1.32 (0.48–3.64) | 0.96 (0.28–3.23) | 0.24 (0.03–1.75) | 1.78 (0.69–4.55) | 1.70 (0.98–2.93) | 0.73 (0.09–5.68) | 1.27 (0.62–2.60) | 1.31 (0.48–3.55) |
| Year of diagnosis | |||||||||
| 1996–2000 | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 2001–2004 | 1.08 (0.78–1.50) | 1.33 (0.95–1.87) | 0.9 (0.69–1.30) | 1.01 (0.74–1.38) | 1.17 (0.82–1.66) | 0.97 (0.80–1.19) | 1.60 (0.87–2.96) | 0.82 (0.66–1.01) | 0.87 (0.64–1.20) |
| 2005–2008 | 1.32 (0.89–1.96) | 1.83 (1.26–2.65) | 0.89 (0.61–1.29) | 1.31 (0.93–1.83) | 0.93 (0.60–1.44) | 1.15 (0.92–1.43) | 2.78 (1.49–5.18) | 0.80 (0.62–1.04) | 0.95 (0.67–1.37) |
| 2009–2012 | 1.61 (0.90–2.86) | 1.48 (0.90–2.44) | 1.01 (0.61–1.67) | 0.97 (0.60–1.57) | 0.71 (0.36–1.39) | 1.20 (0.89–1.64) | 1.95 (0.82–4.62) | 0.97 (0.67–1.41) | 0.60 (0.33–1.11) |
| Histology | |||||||||
| Follicular | 1.01 (0.66–1.55) | 0.89 (0.54–1.48) | 0.79 (0.48–1.30) | 1.19 (0.78–1.81) | 0.86 (0.50–1.49) | 0.98 (0.74–1.30) | 0.60 (0.24–1.54) | 1.07 (0.79–1.43) | 0.88 (0.55–1.39) |
| Papillary | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Stage at diagnosis | |||||||||
| Localized/regional | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Advanced | 1.14 (0.63–2.05) | 1.96 (1.18–3.26) | 1.13 (0.62–2.06) | 0.70 (0.33–1.49) | 1.87 (1.06–3.28) | 1.41 (1.00–1.98) | 1.40 (0.56–3.51) | 0.84 (0.53–1.33) | 0.94 (0.51–1.75) |
| Missing | 1.29 (0.54–3.12) | 2.49 (1.07–5.81) | 0.83 (0.26–2.60) | 1.22 (0.44–3.34) | 0.65 (0.16–2.69) | 0.79 (0.38–1.62) | 0.68 (0.09–5.07) | 0.77 (0.34–1.76) | 1.70 (0.74–3.93) |
| Neighborhood SES | |||||||||
| High | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Low | 0.92 (0.73–1.18) | 1.26 (0.96–1.66) | 1.42 (1.07–1.89) | 1.41 (1.08–1.84) | 1.39 (1.01–1.92) | 1.23 (1.04–1.44) | 1.62 (0.97–2.68) | 1.15 (0.96–1.38) | 1.14 (0.88–1.49) |
| Health insurance | |||||||||
| Private/military | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Public/none | 1.05 (0.72–1.53) | 1.64 (1.20–2.26) | 1.81 (1.32–2.48) | 1.57 (1.16–2.14) | 3.24 (2.33–4.51) | 1.48 (1.22–1.80) | 2.52 (1.51–4.18) | 1.78 (1.43–2.22) | 2.30 (1.70–3.10) |
| Unknown | 1.61 (0.80–3.24) | 0.67 (0.21–2.15) | 3.01 (1.70–5.34) | 0.70 (0.22–2.20) | 2.23 (0.95–5.22) | 1.31 (0.80–2.15) | 1.80 (0.52–6.25) | 1.63 (0.98–2.73) | 0.62 (0.20–1.92) |
| Initial treatment | |||||||||
| Total thyroidectomy plus radioiodine | 1.06 (0.78–1.43) | 1.49 (0.99–2.24) | 0.86 (0.62–1.18) | 1.09 (0.78–1.51) | 0.91 (0.62–1.34) | 1.14 (0.93–1.40) | 0.49 (0.29–0.83) | 0.99 (0.79–1.23) | 0.94 (0.68–1.28) |
| Total thyroidectomy without radioiodine | 0.95 (0.68–1.33) | 1.98 (1.30–3.02) | 0.96 (0.68–1.36) | 1.23 (0.86–1.75) | 1.05 (0.70–1.58) | 1.42 (1.14–1.77) | 1.00 (0.60–1.68) | 1.05 (0.83–1.34) | 0.95 (0.68–1.35) |
| Partial/no thyroidectomy | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Neck reoperationb | |||||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 1.11 (0.65–1.91) | 2.83 (1.93–4.14) | 1.76 (1.09–2.82) | 2.28 (1.51–3.45) | 1.79 (1.05–3.05) | 1.41 (1.05–1.89) | 1.58 (0.66–3.79) | 1.80 (1.29–2.52) | 1.73 (1.07–2.78) |
Incident medical conditions obtained from hospital discharge records.
Adjusted for all variables in the table and overweight/obesity and tobacco use disorder.
Neck reoperation was consider as time-dependent variable.
HR, hazard ratio.
Discussion
In this study of over 12,000 AYA DTC survivors in California, we observed that the development of medical conditions was associated with both sociodemographic and clinical factors. Men, AYAs of black or Hispanic race/ethnicity and those residing in low SES neighborhoods were at an increased risk of a number of medical conditions, consistent with findings of poorer outcomes in these patients.21 Having public or no health insurance at diagnosis or initial treatment was strongly associated with all subsequent medical conditions considered, except second cancers. Furthermore, patients who required neck reoperation, a factor associated with increased mortality,23 were more likely to be diagnosed with endocrine, respiratory, hematologic, and cardiovascular conditions. Our findings confirm prior studies that documented an increased burden of subsequent medical conditions after thyroid cancer diagnosis and expand on them by identifying the higher burden of medical conditions in specific subgroups of DTC survivors, subgroups that should be the focus of survivorship programs to reduce the burden of subsequent disease.
Elevated rates in the diagnosis of medical conditions after a cancer diagnosis may result from factors that include closer surveillance, cancer-associated comorbidities, and treatment-associated adverse effects. Prior studies have established that parathyroid disorders, hypertension, heart disease, and nutritional deficiencies are increased in thyroid cancer survivors compared to matched populations without thyroid cancer.15,19,24 Our study was not designed to assess causality and not limited to disorders directly related to thyroid cancer diagnosis or treatment, but we nonetheless observed variable rates of subsequent endocrine and hematologic diagnoses based on initial treatment. Moreover, neck reoperation was associated with multiple subsequent medical diagnoses. While these diagnoses may have resulted from enhanced contact with hospital care due to subsequent surgery, we previously showed that neck reoperation was associated with a higher risk of thyroid cancer mortality.23
The rate of permanent hypoparathyroidism resulting from total thyroid surgery has been quoted as about 1.7%,9 slightly less than the 10-year cumulative incidence of calcium and phosphorus disorders observed in patients treated with initial total thyroidectomy in this study. A prior study using outpatient claims data identified that the rates of postoperative hypoparathyroidism parallel the intensity of screening for and diagnosis of thyroid cancer.24 Additional factors, such as the extent of central neck dissection, previously associated with increasing rates of associated hypoparathyroidism,27 may account for subgroups with higher rates. Those diagnosed with DTC between 2005 and 2008 had a higher risk of calcium and phosphorus disorders, a period during which routine central neck dissection was recommended for all papillary thyroid cancers.28 Consistent with this association between more extensive neck surgery and incident parathyroid disorders, we observed that more advanced initial stage and neck reoperation were strong predictors of subsequent calcium and phosphorus disorders. We did not observe an impact of the extent of initial treatment on the prespecified respiratory or cardiovascular disorders, despite potential impact of the extent of surgery on vocal cord dysfunction and radioiodine on pulmonary fibrosis.10,12
Long-term use of levothyroxine has pleiotropic effects on the cardiovascular system, including increasing the heart rate, cardiac contractility, and blood pressure.29 In patients with a suppressed TSH, this is associated with an increased risk of cardiovascular disease.30,31 We confirmed a high cumulative incidence of subsequent hypertension and other cardiovascular diseases seen in a prior study of DTC patients from Utah.15 We also demonstrate that these rates are particularly high in the black population, which has more than twice the risk of hypertension than the non-Hispanic white population adjusting for other demographic and treatment factors, reaching a cumulative incidence of 11.3% at 10 years.
On average, survivors of AYA cancer report higher rates of health care utilization compared to their peers,32 potentially resulting in an increase in the number of health conditions being diagnosed in this study. Nonetheless, AYA cancer survivors with no insurance are more likely to forego subsequent medical care than those with insurance.33,34 We did not have access to data regarding changes in insurance coverage over time. However, prior studies suggest that the rate of the uninsured or publicly insured increases in cancer survivors over time,33,35,36 suggesting that insurance-related barriers to care may increase over time. Indeed, among a subset of patients from our study (n = 4527) with insurance information after 2 years, we observed that 22% had public or no insurance, somewhat higher than the 15% within this subgroup at diagnosis. Despite established barriers to care that may increase during survivorship, we found that AYA DTC survivors with public or no insurance at diagnosis were more likely to have an incident medical diagnosis recorded from a hospitalization in this study. Therefore, this group is particularly amenable to policy-based interventions to reduce the burden of subsequent medical conditions.
An important finding in our study is that living in a low SES neighborhood is associated with higher rates of multiple subsequent diagnoses. With our study design, we cannot specify the relative contribution of differential initial treatment factors or administration of subsequent care on the overall disparities in the development of subsequent diagnoses among SES groups. An impact of low SES on multiple health outcomes exists absent a cancer diagnosis.37,38 Nonetheless, the impact of cancer diagnosis on subsequent financial, psychological, and emotional well-being has been extensively studied.34,39–44 Our results are consistent with prior work supporting the hypothesis that lower SES AYA survivors of cancer are more susceptible to conditions that are the result of reduced routine preventative care, providing a target for interventions to reduce the burden of subsequent disease.
A major advantage of this study is the inclusion of a large population of AYA DTC survivors that reflects a degree of racial/ethnic diversity not available in population-based studies from other states. Indeed, one of the important findings of this study is that the rates of incident medical conditions vary markedly by race/ethnicity. We focused on medical conditions that were obtained from hospital discharge records such that differences between sociodemographic and treatment groups may have resulted from nonrandom rates of hospitalization. In addition, we were unable to assess medical conditions that were not captured in hospital discharge records due to care being received in the outpatient setting. Moreover, we were limited by the inability to fully assess the extent of nodal dissection with initial surgery, the activity of radioiodine administered, and the extent of TSH suppression. Finally, because our observational cohort exists entirely of patients with a DTC diagnosis and no similar population without a cancer diagnosis is available, we are unable to quantify the attributable risk of thyroid cancer diagnosis and treatment on the resultant incident medical conditions. Nonetheless, the findings of our study are fully applicable to large populations of AYA DTC survivors and identify those who are at risk for medical conditions that result in subsequent inpatient care.
In conclusion, the diagnosis of new serious medical conditions occurs frequently after AYA DTC identification and treatment. The risk of specific diagnoses vary by race/ethnicity and sex. Asian/Pacific Islanders are more likely to develop subsequent cancers, while Hispanics and blacks were more likely to develop diabetes and hematologic cytopenia. Male and black AYAs had a higher risk of cardiovascular diagnoses. Disorders of calcium and phosphorus metabolism diagnoses are particularly common after more intensive initial surgery or after subsequent neck surgery. Low SES and public or no medical insurance are strong predictors for developing subsequent medical conditions and these groups should be a focus for survivorship programs to reduce the burden of disease in AYA DTC survivors.
Disclaimer
The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Rich and Weissman Family Lymphoma and Survivorship Fund St. Baldrick's Research Grant. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention in this study was supported by the California cooperative agreement 5NU58DP003862-04/DP003862; the National Cancer Institute Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute.
References
- 1. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317–22. [DOI] [PubMed] [Google Scholar]
- 2. Enewold L, Zhu K, Ron E, et al. . Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomark Prev. 2009;18(3):784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaccarella S, Franceschi S, Bray F, et al. . Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614–7. [DOI] [PubMed] [Google Scholar]
- 4. Lim H, Devesa SS, Sosa JA, et al. . Trends in thyroid cancer incidence and mortality in the United States, 1974–2013. JAMA. 2017;317(13):1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qian ZJ, Jin MC, Meister KD, et al. . Pediatric thyroid cancer incidence and mortality trends in the United States. JAMA Otolaryngol Head Neck Surg. 2019;145:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Cancer Institute. SEER cancer statistics factsheets: thyroid cancer. 2019. Accessed March 26, 2019. from: www.seer.cancer.gov/statfacts/html/thyro.html
- 7. Haugen BR, Alexander EK, Bible KC, et al. . 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergenfelz A, Jansson S, Kristoffersson A, et al. . Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393(5):667–73. [DOI] [PubMed] [Google Scholar]
- 9. Rosato L, Avenia N, Bernante P, et al. . Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28(3):271–6. [DOI] [PubMed] [Google Scholar]
- 10. Papaleontiou M, Hughes DT, Guo C, et al. . Population-based assessment of complications following surgery for thyroid cancer. J Clin Endocrinol Metab. 2017;102(7):2543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Semrad TJ, Semrad AM, Farwell DG, et al. . Initial treatment patterns in younger adult patients with differentiated thyroid cancer in California. Thyroid. 2015;25(5):509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fard-Esfahani A, Emami-Ardekani A, Fallahi B, et al. . Adverse effects of radioactive iodine-131 treatment for differentiated thyroid carcinoma. Nucl Med Commun. 2014;35(8):808–17. [DOI] [PubMed] [Google Scholar]
- 13. Jeong SY, Kim HW, Lee S-W, et al. . Salivary gland function 5 years after radioactive iodine ablation in patients with differentiated thyroid cancer: direct comparison of pre- and postablation scintigraphies and their relation to xerostomia symptoms. Thyroid. 2013;23(5):609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu JX, Young S, Ro K, et al. . Reproductive outcomes and nononcologic complications after radioactive iodine ablation for well-differentiated thyroid cancer. Thyroid. 2015;25(1):133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park J, Blackburn BE, Ganz PA, et al. . Risk factors for cardiovascular disease among thyroid cancer survivors: findings from the Utah cancer survivors study. J Clin Endocrinol Metab. 2018;103(7):2468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cho YY, Lim J, Oh C-M, et al. . Elevated risks of subsequent primary malignancies in patients with thyroid cancer: a nationwide, population-based study in Korea. Cancer. 2015;121(2):259–68. [DOI] [PubMed] [Google Scholar]
- 17. Bright CJ, Reulen RC, Winter DL, et al. . Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study): a population-based, cohort study. Lancet Oncol. 2019;20:531–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee JS, DuBois SG, Coccia PF, et al. . Increased risk of second malignant neoplasms in adolescents and young adults with cancer. Cancer. 2016;122(1):116–23. [DOI] [PubMed] [Google Scholar]
- 19. Blackburn BE, Ganz PA, Rowe K, et al. . Aging-related disease risks among young thyroid cancer survivors. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rugbjerg K, Olsen JH. Long-term risk of hospitalization for somatic diseases in survivors of adolescent or young adult cancer. JAMA Oncol. 2016;2(2):193–200. [DOI] [PubMed] [Google Scholar]
- 21. Keegan TH, Grogan RH, Parsons HM, et al. . Sociodemographic disparities in differentiated thyroid cancer survival among adolescents and young adults in California. Thyroid. 2015;25(6):635–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yost K, Perkins C, Cohen R, et al. . Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–11. [DOI] [PubMed] [Google Scholar]
- 23. Semrad TJ, Keegan THM, Semrad A, et al. . Predictors of neck reoperation and mortality after initial total thyroidectomy for differentiated thyroid cancer. Thyroid. 2018;28(9):1143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ahn SV, Lee J-H, Bove-Fenderson EA, et al. . Incidence of hypoparathyroidism after thyroid cancer surgery in South Korea. JAMA. 2019;322(24):2441–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin G, So Y, Johnston G. Analyzing survival data with competing risks using SAS® software. Proceedings of the SAS® Global Forum 2012 Conference. SAS Institute, Inc., Cary, NC, 2012.
- 26. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–54. [Google Scholar]
- 27. Giordano D, Valcavi R, Thompson GB, et al. . Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid. 2012;22(9):911–7. [DOI] [PubMed] [Google Scholar]
- 28. Cooper DS, Doherty GM, Haugen BR, et al. . Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109–42. [DOI] [PubMed] [Google Scholar]
- 29. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501–9. [DOI] [PubMed] [Google Scholar]
- 30. Flynn RW, Bonellie SR, Jung RT, et al. . Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab. 2010;95(1):186–93. [DOI] [PubMed] [Google Scholar]
- 31. Parker WA, Edafe O, Balasubramanian SP. Long-term treatment-related morbidity in differentiated thyroid cancer: a systematic review of the literature. Pragmatic Obs Res. 2017;8:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kirchhoff AC, Kaul S, Fluchel M, et al. . Healthcare utilization and quality among survivors of adolescent and young adult cancer. J Clin Oncol. 2016;34(3_Suppl):21. [Google Scholar]
- 33. Keegan THM, Tao L, DeRouen MC, et al. . Medical care in adolescents and young adult cancer survivors: what are the biggest access-related barriers? J Cancer Surviv Res Pract. 2014;8(2):282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kirchhoff AC, Lyles CR, Fluchel M, et al. . Limitations in health care access and utilization among long-term survivors of adolescent and young adult cancer. Cancer. 2012;118(23):5964–72. [DOI] [PubMed] [Google Scholar]
- 35. Kaul S, Fluchel M, Spraker-Perlman H, et al. . Health care experiences of long-term survivors of adolescent and young adult cancer. Support Care Cancer 2016;24(9):3967–77. [DOI] [PubMed] [Google Scholar]
- 36. Parsons HM, Schmidt S, Harlan LC, et al. . Young and uninsured: insurance patterns of recently diagnosed adolescent and young adult cancer survivors in the AYA HOPE study. Cancer. 2014;120(15):2352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schultz WM, Kelli HM, Lisko JC, et al. . Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. 2018;137(20):2166–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grintsova O, Maier W, Mielck A. Inequalities in health care among patients with type 2 diabetes by individual socio-economic status (SES) and regional deprivation: a systematic literature review. Int J Equity Health. 2014;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yabroff KR, Dowling EC, Guy GPJ, et al. . Financial hardship associated with cancer in the United States: findings from a population-based sample of adult cancer survivors. J Clin Oncol. 2016;34(3):259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Alvarez E, Keegan T, Johnston EE, et al. . Adolescent and young adult oncology patients: disparities in access to specialized cancer centers. Cancer. 2017;123(13):2516–23. [DOI] [PubMed] [Google Scholar]
- 41. Oeffinger KC. Longitudinal risk-based health care for adult survivors of childhood cancer. Curr Probl Cancer. 2003;27(3):143–67. [DOI] [PubMed] [Google Scholar]
- 42. Salsman JM, Bingen K, Barr RD, et al. . Understanding, measuring, and addressing the financial impact of cancer on adolescents and young adults. Pediatr Blood Cancer. 2019;66(7):e27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Landwehr MS, Watson SE, Macpherson CF, et al. . The cost of cancer: a retrospective analysis of the financial impact of cancer on young adults. Cancer Med. 2016;5(5):863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Overholser L, Kilbourn K, Liu A. Survivorship issues in adolescent and young adult oncology. Med Clin North Am. 2017;101(6):1075–84. [DOI] [PubMed] [Google Scholar]