Abstract
Objective
To assess whether patients with aortic valve stenosis (AS) with elevated lipoprotein(a) (Lp(a)) are characterised by increased valvular calcification activity compared with those with low Lp(a).
Methods
We performed 18F-sodium fluoride (18F-NaF) positron emission tomography/CT in patients with mild to moderate AS (peak aortic jet velocity between 2 and 4 m/s) and high versus low Lp(a) (>50 mg/dL vs <50 mg/dL, respectively). Subjects were matched according to age, gender, peak aortic jet velocity and valve morphology. We used a target to background ratio with the most diseased segment approach to compare 18F-NaF uptake.
Results
52 individuals (26 matched pairs) were included in the analysis. The mean age was 66.4±5.5 years, 44 (84.6%) were men, and the mean aortic valve velocity was 2.80±0.49 m/s. The median Lp(a) was 79 (64–117) mg/dL and 7 (5–11) mg/dL in the high and low Lp(a) groups, respectively. Systolic blood pressure and low-density-lipoprotein cholesterol (corrected for Lp(a)) were significantly higher in the low Lp(a) group (141±12 mm Hg vs 128±12 mm Hg, 2.5±1.1 mmol/L vs 1.9±0.8 mmol/L). We found no difference in valvular 18F-NaF uptake between the high and low Lp(a) groups (3.02±1.26 vs 3.05±0.96, p=0.902). Linear regression analysis showed valvular calcium score to be the only significant determinant of valvular 18F-NaF uptake (β=0.63; 95% CI 0.38 to 0.88 per 1000 Agatston unit increase, p<0.001). Lp(a) was not associated with 18F-NaF uptake (β=0.17; 95% CI −0.44 to 0.88, p=0.305 for the high Lp(a) group).
Conclusion
Among patients with mild to moderate AS, calcification activity is predominantly determined by established calcium burden. The results do not support our hypothesis that Lp(a) is associated with valvular 18F-NaF uptake.
Keywords: aortic valve stenosis, positron emission tomography computed tomography
Introduction
Aortic valve stenosis (AS) is one of the most frequent cardiovascular diseases in the Western world.1 Although risk factors for AS are similar to those for atherosclerosis, previous randomised trials with statins or renin-angiotensin-aldosterone inhibitors have not been able to influence the progression of AS.2 Consequently, current management revolves around a wait-and-see approach until the development of severe AS warrants surgical or transcatheter valve replacement.3
Lipoprotein(a) (Lp(a)) has been established as an independent, likely causal risk factor for incident AS.4–6 Interventions aimed at lowering Lp(a), including proprotein convertase subtilisin/kexin type 9 inhibitors and apolipoprotein(a) antisense therapies, may be effective in delaying or preventing AS progression.7 However, the optimal timing of medical intervention in AS remains unresolved. The failure of previous statin trials to delay haemodynamic progression is often attributed to initiation at a disease stage too advanced to be amenable for intervention, at which point other disease mechanisms drive disease progression independent of hypercholesterolaemia.
Valvular 18F-sodium fluoride (NaF) uptake assessed by positron emission tomography (PET)/CT is robustly correlated with progression of calcification.8 A recent post-hoc analysis of longitudinal imaging studies reported that elderly patients with advanced AS and higher Lp(a) levels were characterised by higher valvular uptake of 18F-NaF and faster disease progression.9 These data support the concept of lowering Lp(a) in patients with established AS to mitigate disease progression. Whether earlier intervention in the disease process could be beneficial remains unexplored.
In the current study, we hypothesised that patients with mild to moderate AS and significantly elevated Lp(a) levels (>50 mg/dL) are characterised by a higher degree of valvular calcification activity than those with lower Lp(a) levels. As such, we performed a matched case–control study where we assessed valvular calcification activity by using 18F-NaF PET/CT in patients with mild to moderate AS with higher versus lower Lp(a) levels.
Methods
Patient selection
Participants were recruited from a biobank, consisting of 190 patients with mild to moderate AS who were previously recruited from the cardiology outpatient clinics of the Amsterdam University Medical Centers and Cardiologie Centra Nederland. Subjects were eligible for the study if they were aged between 50 and 80 years and had a peak aortic jet velocity between 2 and 4 m/s. Exclusion criteria were severe AS (peak aortic jet velocity >4 m/s), aortic valve area <1.0 cm2, history of radiotherapy of the thorax, history of rheumatic fever, estimated glomerular filtration rate <30 mL/min, hyperparathyroidism, Paget’s disease and/or any contraindication to PET/CT imaging. All eligible patients with high Lp(a) (>50 mg/dL) were asked to participate, who were then matched to controls with low Lp(a) levels according to sex, age, aortic valve morphology and peak aortic jet velocity. Detailed questionnaires regarding demographics, general health status, medical history, family history, risk factors, medication, blood pressure, height and weight were taken during the biobank study visit. Echocardiography was performed within 6 months of PET/CT imaging. All subjects gave written informed consent.
Laboratory measurements
Baseline blood samples including Lp(a) measurements were available from a previous biobank study. Haematology, chemistry and lipid panels were determined according to standardised operating procedures in a core laboratory. Low-density-lipoprotein cholesterol (LDL-C) was calculated using the Friedewald formula.10 Lp(a) was measured in all patients on serum samples using the kringle IV type 2 number independent Randox immunoassay (Randox Laboratories, UK). We corrected LDL-C for Lp(a) by subtracting 0.15×Lp(a) mass from the LDL-C mass.11
Echocardiography
Echocardiography was performed by the same research cardiologist if not performed within 6 months prior to PET/CT imaging, using a dedicated echocardiography device (Vivid E95, GE Healthcare, Chicago, USA). The aortic valve-specific echocardiogram included determination of aortic valve morphology, peak aortic jet velocity, transvalvular gradients and aortic valve area.
Image acquisition and analysis
ECG-gated 18F-NaF PET and contrast-enhanced CT angiography were performed using a hybrid scanner (Biograph mCT, Siemens, Medical Systems, Erlangen, Germany) 60 min after infusion of 125 MBq 18F-NaF. If resting heart rate was >65 beats per minute, metoprolol 50 or 100 mg was administered according to clinical protocols. PET data were reconstructed using the Siemens Ultra HD reconstruction algorithm with correction for dead time, attenuation, random coincidences and scatter. 18F-NaF PET/CT images were assessed using FusionQuant V.1.20.05.14 (Cedars-Sinai, California, USA) using a standardised method.12 13 After fusing diastolic PET images with the contrast-enhanced CT, an en-face view was established and the PET window level adjusted to allow visual determination of the point of maximal specific valvular uptake. Automated correction for cardiac motion is possible and fully integrated within FusionQuant, and this was performed with an anatomically guided registration algorithm according to previously published methods.14 Briefly, a spherical region of interest was drawn to include the entire aortic valve. A non-linear registration algorithm, radially constrained around the aortic valve, was used to align PET images to the diastolic gate. The motion-corrected gates were then summed to form a motion-free image containing all the PET counts. A three-dimensional polyhedron, 6 mm in height and contoured to the valve frame, was centred on this point and formed the volume of interest. 18F-NaF blood pool uptake was measured in the right atrium with a cylinder 10 mm in radius by 10 mm in height. Where present, pacing leads in the right atrium were excluded. In the control cohort, the valvular region of interest was centred on the valve leaflets in the z-axis, ensuring the lower border included the leaflet bases. The aortic valve maximum target to background ratio (TBRmax), a measure of the point of most intense 18F-NaF uptake within the volume of interest, was calculated as the valvular maximum standardised uptake value (SUVmax) divided by the atrial blood pool mean standardised uptake value (SUVmean). TBRmean, a measure of the average 18F-NaF uptake in the volume of interest, was calculated as the valvular SUVmean divided by the blood pool SUVmean. Within the volume of interest, we measured the aortic valve microcalcification activity (AVMA), representing the overall disease activity in the aortic valve and based on both the volume and intensity of 18F-NaF PET activity within it (similar in principle to coronary microcalcification activity).15 AVMA was defined as the integrated activity in SUV units exceeding the corrected background blood pool mean SUV plus 2 SD (right atrium activity). CT aortic valve calcium (AVC) scoring according to Agatston methodology16 was performed using dedicated software on axial views (Syngo.via, Siemens, Medical Systems). All measurements were performed by experienced readers who were blinded to Lp(a) levels.
Statistical analysis
Data are presented as mean±SD for normally distributed variables, median with IQR for non-normally distributed variables, and number (percentage) for categorical variables. Between group testing was performed using t-test for normally distributed data, Mann-Whitney U test for non-normally distributed data, and χ2 or Fisher’s exact test for categorical data. Based on previous PET/CT studies for the assessment of arterial wall inflammation,17 we conservatively estimated the expected mean difference in valvular 18F-NaF uptake between high and low Lp(a) groups to be 15%. To detect this difference with α=0.05% and 80% power, we aimed to enrol 25 patients per group. Multivariable models accounting for age, sex, AVC score and significantly different baseline clinical parameters (p<0.05) were used to assess differences in valvular TBR between high and low Lp(a) groups. Exploratory linear regression analyses were performed to assess whether increasing Lp(a) within the high Lp(a) group was associated with active calcification, and to evaluate if significantly different baseline characteristics (if any) were related to active calcification in the low Lp(a) group. AVC scores were log-transformed. A two-sided p value <0.05 was considered statistically significant. The statistical analyses were performed using RStudio software (V.4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
Patient and public involvement
Participants were not involved in determining the research question or outcome measures, nor were they involved in recruitment, design or implementation of the study. Participants were not asked for advice on the interpretation of results.
Results
Study population
In total, 58 patients were included between February 2018 and October 2020. One patient withdrew prior to PET/CT imaging, one was excluded from further analysis due to absence of aortic valve calcification, and one was excluded due to a history of radiation for breast cancer. In addition, we were unable to find an appropriate match for three participants. A total of 52 patients, consisting of 26 matched pairs, were included in the current analysis. There were no missing data. Baseline characteristics are listed in table 1. The mean age at the date of inclusion was 66.0±4.2 years. The majority of participants were men (86.7%). Five patients in each group had bicuspid valve morphology.
Table 1.
Baseline characteristics
| High Lp(a) (n=26) | Low Lp(a) (n=26) | P value | |
| Clinical characteristics | |||
| Age, years | 66.3±5.6 | 66.5±5.6 | 0.934* |
| Male gender | 22 (84.6) | 22 (84.6) | >0.999† |
| Body mass index, kg/m2 | 26.9±4.5 | 28.5±4.2 | 0.316* |
| Systolic blood pressure, mm Hg | 128±12 | 141±12 | <0.001* |
| Diastolic blood pressure, mm Hg | 79±12 | 85±11 | 0.057* |
| Ischaemic heart disease | 6 (23.1) | 2 (7.7) | 0.248‡ |
| Hypertension | 20 (76.9) | 20 (76.9) | >0.999† |
| Diabetes mellitus | 3 (11.5) | 7 (26.9) | 0.291‡ |
| Smoking | |||
| Never | 8 (30.8) | 9 (34.6) | 0.872‡ |
| Former | 12 (46.2) | 13 (50) | |
| Active | 6 (23.1) | 4 (15.4) | |
| Use of lipid-lowering therapy | 19 (73.1) | 16 (61.5) | 0.554† |
| Use of antihypertensive(s) | 18 (69.2) | 19 (73.1) | >0.999† |
| Laboratory values | |||
| C reactive protein, mg/dL | 1.1 (0.5–4.8) | 1.3 (0.9–2.3) | 0.521* |
| Creatinine, mmol/L | 87±20 | 87±18 | 0.903* |
| Urea, mmol/L | 6.8±2.3 | 7.0±2.1 | 0.775* |
| Calcium, mmol/L | 2.41±0.09 | 2.42±0.08 | 0.713* |
| Phosphate, mmol/L | 0.94±0.20 | 0.94±0.16 | 0.933* |
| Total cholesterol, mmol/L | 4.43±0.94 | 4.85±1.13 | 0.151* |
| HDL-cholesterol, mmol/L | 1.54±0.34 | 1.62±0.72 | 0.606* |
| LDL-cholesterol, mmol/L | 2.3±0.8 | 2.6±1.1 | 0.361* |
| Corrected LDL-cholesterol, mmol/L | 1.9±0.8 | 2.5±1.3 | 0.035* |
| Triglycerides, mmol/L | 1.09 (0.70–1.55) | 1.29 (1.05–2.11) | 0.090§ |
| Lp(a), mg/dL | 79 (64–112) | 5 (3–10) | <0.001§ |
| Echocardiographic parameters | |||
| Bicuspid aortic valve | 5 (19.2) | 5 (19.2) | >0.999† |
| Peak aortic jet velocity, m/s | 2.77±0.55 | 2.83±0.42 | 0.689* |
| Peak aortic valve gradient, mm Hg | 31±13.55 | 30.85±10.68 | 0.964* |
| Mean aortic valve gradient, mm Hg | 17.88±8.31 | 17.88±6.96 | 0.998* |
| Aortic valve area, cm2 | 1.77±0.64 | 1.62±0.42 | 0.345* |
Data are presented as mean±SD, median (IQR) or number (percentage).
*Hypothesis tested by t-test.
†Hypothesis tested by χ2.
‡Hypothesis tested by Fisher’s exact test.
§Hypothesis tested by Mann-Whitney U test.
HDL, high-density-lipoprotein; LDL, low-density-lipoprotein; Lp(a), lipoprotein(a).
Higher and lower Lp(a) groups
Higher (>50 mg/dL) and lower (<50 mg/dL) Lp(a) groups were well matched for age, sex, aortic valve morphology and peak aortic jet velocity (table 1). There was a more than tenfold difference in Lp(a) levels between the two groups. The median Lp(a) in the high group was 79 (IQR 64–117) mg/dL, compared with 7 (IQR 5–11) mg/dL in the lower Lp(a) group. Corrected LDL-C levels were significantly higher in the lower Lp(a) group (1.9±0.8 mmol/L in the high vs 2.5±1.1 mmol/L in the low Lp(a) group, p=0.035), as was systolic blood pressure (128±12 mm Hg in the high vs 141±12 mm Hg in the low Lp(a) group, p<0.001).
Valvular imaging parameters
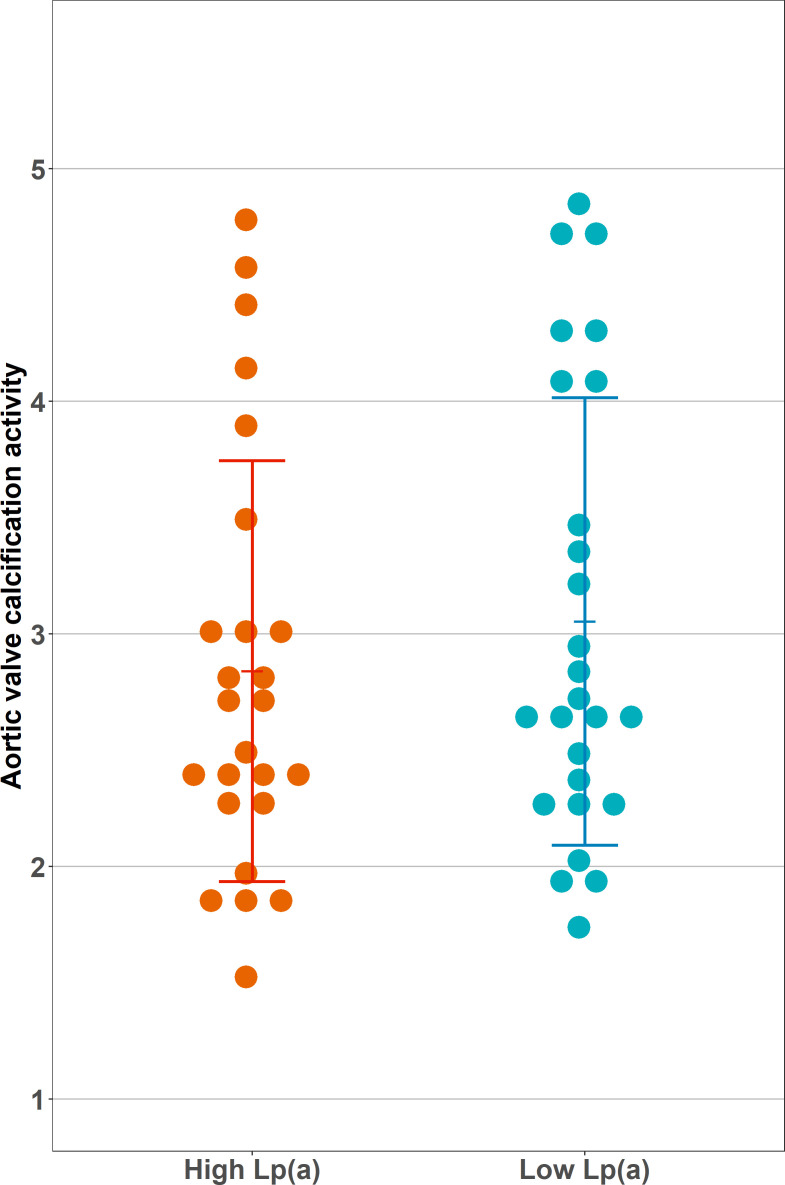
All valvular imaging parameters are listed in table 2. The CT AVC scores in the high and low Lp(a) groups were comparable between the groups (1388 Agatston units (AU) (450–2424) in the high vs 1173 (927–1628) AU in the low Lp(a) group, p=0.839). In contrast to our hypothesis, we did not observe a significant difference in calcification activity between the groups: the aortic valve TBRmax was 3.02±1.26 in the high Lp(a) group compared with 3.05±0.96 in the low Lp(a) group (p=0.902). The TBRmean showed comparable results, being 1.84±0.42 in the high Lp(a) group vs 1.86±0.49 in the low Lp(a) group (p=0.863). SUV values also did not differ significantly between the groups nor did AVMA. Figure 1 shows the distribution of valvular 18F-NaF uptake in both groups.
Table 2.
Aortic valve imaging parameters
| High Lp(a) (n=26) | Low Lp(a) (n=26) | P value | |
| Aortic valve calcium score (AU) | 1388 (450–2424) | 1173 (927–1628) | 0.839* |
| Aortic valve TBRmax | 3.02±1.26 | 3.05±0.96 | 0.902† |
| Aortic valve TBRmean | 1.84±0.42 | 1.86±0.49 | 0.863† |
| Aortic valve SUVmax | 3.29±1.38 | 3.10±0.79 | 0.553† |
| Aortic valve SUVmean | 1.87±0.40 | 2.00±0.64 | 0.399† |
| Aortic valve microcalcification activity | 8.30±3.58 | 6.96±2.68 | 0.144† |
| Right atrium SUVmean | 1.08±0.32 | 1.04±0.24 | 0.640† |
Data are presented as mean±SD or median (IQR).
*Hypothesis tested by Mann-Whitney U test.
†Hypothesis tested by t-test.
AU, Agatston units; Lp(a), lipoprotein(a); SUVmax, maximum standardised uptake value; SUVmean, mean standardised uptake value; TBRmax, maximum target to background ratio; TBRmean, mean target to background ratio.
Figure 1.
Aortic valve calcification activity stratified by lipoprotein(a) (Lp(a)) group. Depicted is the calcification activity of the aortic valve, measured as maximum target to background ratio. This was calculated by dividing the valvular maximum standardised uptake by the blood pool mean standardised uptake value. There was no significant difference in calcification activity between high and low Lp(a) groups (3.02±1.26 vs 3.05±0.96, p=0.902).
Determinants of valvular calcification activity
In univariable regression analysis, only AVC score and male sex were significantly associated with valvular 18F-NaF uptake, whereas age, Lp(a) group, LDL-C and systolic blood pressure were not. Univariable analysis with only CT AVC score as determinant of valvular 18F-NaF uptake showed a better fit than the multivariable model with age, sex, Lp(a) group, systolic blood pressure and corrected LDL-C (adjusted R2=0.40 for univariable and adjusted R2=0.35 for multivariable model). In multivariable linear regression analysis, the AVC score was the only determinant of valvular 18F-NaF uptake (β=0.63 per 1000 increase in AU, 95% CI 0.38 to 0.88, p<0.001). Lp(a) group was not significantly associated with valvular 18F-NaF uptake (β=0.17 for the high Lp(a) group, 95% CI −0.44 to 0.78, p=0.305), nor were age, sex, systolic blood pressure and corrected LDL-C (table 3). As an exploratory analysis, we assessed whether increasing Lp(a) was associated with more calcification activity in the high Lp(a) group only, and whether increasing blood pressure and LDL-C were drivers of calcification activity in the low Lp(a) group, but CT AVC score remained the only predictor (online supplemental table 1).
Table 3.
Linear regression analysis for valvular 18F-NaF uptake
| Univariable analysis β (95% CI) |
P value | Multivariable analysis β (95% CI) |
P value | |
| Intercept | 2.65 (−2.65 to 6.23) | 0.217 | ||
| Aortic valve calcium score (per 1000 AU increase) | 0.60 (0.39 to 0.81) | <0.001 | 0.63 (0.38 to 0.88) | <0.001 |
| Age (per 10-year increase) | 0.23 (−0.34 to 0.80) | 0.416 | −0.25 (−0.74 to 0.24) | 0.305 |
| Male sex | 0.87 (0.04 to 1.70) | 0.041 | 0.06 (−0.77 to 0.88) | 0.891 |
| High lipoprotein(a) group | 0.04 (−0.59 to 0.66) | 0.902 | 0.17 (−0.44 to 0.78) | 0.573 |
| Systolic blood pressure (per 10 mm Hg increase) | 0.05 (−0.18 to 0.29) | 0.639 | 0.10 (−0.12 to 0.32) | 0.387 |
| Corrected LDL-C (per mmol/L increase) | 0.19 (−0.11 to 0.50) | 0.216 | −0.07 (−0.36 to 0.23) | 0.648 |
Data are standardised coefficients (β) with 95% CI.
Adjusted R2=0.40 for the univariable analysis with aortic valve calcium score and adjusted R2=0.35 for the multivariable analysis.
AU, Agatston units; LDL-C, low-density-lipoprotein cholesterol; NaF, sodium fluoride.
heartjnl-2021-319804supp001.pdf (47.5KB, pdf)
Discussion
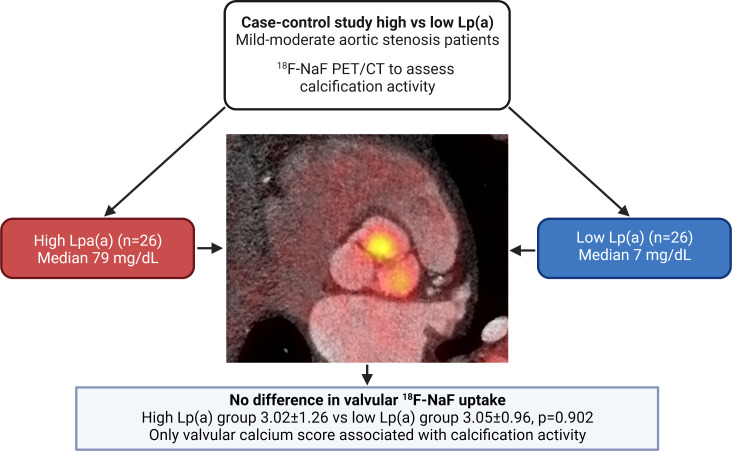
In our study, we did not find a significant difference in valvular calcification activity, assessed with 18F-NaF PET/CT, in patients with mild to moderate AS and higher levels of Lp(a), compared with patients with lower Lp(a), when matched for age, sex and AS severity (figure 2). Since patients with lower Lp(a) were characterised by a significant increase in systolic blood pressure and LDL-C, both known risk factors for AS, this may have obscured a potential effect of Lp(a). However, we observed the CT AVC score to be a highly significant predictor of valvular 18F-NaF uptake in patients with mild to moderate AS. These results suggest that initiating risk factors may lose their relative importance in more advanced stages of AS, which seems to be primarily driven by valvular calcific burden.
Figure 2.
Lp(a) has no major impact on calcification activity in patients with mild to moderate aortic valve stenosis. In this case–control study consisting of matched patients with aortic stenosis with high versus low Lp(a), we observed comparable calcification activity in both groups. Aortic valve calcium score was the only variable associated with 18F-NaF uptake in linear regression analysis (β=0.60 per 1000 Agatston unit increase, 95% CI 0.39 to 0.81). Lp(a), lipoprotein(a); NaF PET/CT, sodium fluoride positron emission tomography/CT.
Lp(a) and initiation of aortic valve disease
Lp(a) has been demonstrated to accumulate within the aortic valve leaflets of patients with AS, co-localising with calcified areas. Mechanistically, Lp(a) was shown to cause osteogenic differentiation in valvular interstitial cells via increased phosphorylation of several kinases such as p38 mitogen-activated protein kinase and glycogen synthase kinase 3 beta.18 In support of a potent calcification response of Lp(a), Després and colleagues19 observed higher valvular 18F-NaF uptake in healthy individuals with high Lp(a) levels of whom the majority had no observable calcifications on CT. In our study, valvular 18F-NaF uptake was approximately twofold higher than in the healthy volunteers in the study by Després and colleagues,19 but we did not observe a difference in 18F-NaF uptake between patients with markedly elevated versus low Lp(a) levels. The discrepancy between these studies likely relates to the presence of established calcifications in the aortic valves of all our patients as opposed to none to negligible calcifications in Després and colleagues’19 study. In support, CT AVC score was highly predictive of 18F-NaF uptake in patients with AS, implying that the contribution of Lp(a) is lower in more advanced disease stages of AS.20
Lp(a) and progression of established AS
In previous studies, Lp(a) has also been associated with faster AS progression. This was first demonstrated in a post-hoc analysis of the Aortic Stenosis Progression Observation: Measuring Effects of Rosuvastatin (ASTRONOMER) trial, which evaluated the effect of statin treatment in relatively young patients (mean age 58 years) with mild to moderate AS.21 Elevated Lp(a) levels were associated with more rapid haemodynamic AS progression on echocardiography over a median follow-up of 3.5 years, especially in younger patients (<57 years, median). These data suggested that as patients get older, other risk factors or disease mechanisms may come into play as drivers of disease progression. A subsequent post-hoc analysis of two longitudinal imaging studies (Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE)/Ring of Fire) also supported a role of Lp(a) in older patients (mean age 70 years) with more advanced AS.9 Patients in the top Lp(a) tertile (>35 mg/dL) showed higher valvular 18F-NaF uptake at baseline, which associated with faster CT AVC score progression and a higher HR for valve replacement and cardiovascular disease during follow-up, as compared with patients in the lower tertiles of Lp(a). Although we selected patients with mild to moderate AS and much higher Lp(a) levels in this study, we could not establish a difference in 18F-NaF uptake between high and low Lp(a) patients.
What drives progression of established aortic valve disease?
After strict matching for AS severity, age and sex, we did observe significantly higher blood pressure and LDL-C in patients with AS with low Lp(a). It is possible that the significantly higher blood pressure and LDL-C in the low Lp(a) group obscured the effect of Lp(a) on valvular disease activity. Nevertheless, we did not find associations between blood pressure, LDL-C and valvular 18NaF uptake. Additionally, we did not find increasing Lp(a) levels within the high Lp(a) group to be associated with increased calcification activity, nor were systolic blood pressure or LDL-C associated with calcification activity in the low Lp(a) group. The CT AVC score was the only determinant of valvular calcification activity, even though we included patients with milder disease (peak aortic jet velocity 2.8 m/s) as compared with the previous imaging studies. While at odds with the Lp(a) literature to date, this observation is consistent with other data demonstrating that while traditional cardiovascular risk factors (eg, LDL-cholesterol) are associated with new-onset incident AVC, they show no relation with disease progression after correction for baseline AVC score.22 23 Further studies are now required to resolve this apparent discrepancy and also whether the relationship between Lp(a) and disease progression depends on baseline AS severity.
Study limitations
This study has several limitations that deserve consideration. First, our sample size was small and only able to detect a 15% or larger difference in valvular 18F-NaF uptake between groups. This modest sample size also led to the linear regression analyses having more predictors than would be appropriate for the number of observations. However, as the beta for the high Lp(a) group in our linear regression analysis was only 0.17, which reflects 5.6% of total valvular 18F-NaF uptake, it is unlikely that a larger sample size would have been able to show a significant relationship between Lp(a) and calcification activity. Our study does not refute that Lp(a) causes calcification, which has been clearly established, but its effect in mild to moderate AS may be less pronounced than initially expected. Another important limitation was the higher burden of competing risk factors (systolic blood pressure and LDL-C) in the low Lp(a) group. Our study was designed to study the intrinsic impact of Lp(a) on 18F-NaF uptake, and therefore we applied very strict matching on age, sex and AS severity. Apparently, patients with low Lp(a) required a higher burden of alternative risk factors in order to achieve similar AS severity, which may have masked an association between Lp(a) and 18F-NaF uptake. However, the robust relationship between CT AVC score and valvular 18F-NaF uptake, combined with the absence of an association between Lp(a), blood pressure, LDL-C and calcification activity, makes this less likely. Nevertheless, we recognise that our study did not have sufficient power to adequately investigate the effects of these competing risk factors on calcification activity. Finally, we do not have data on the progression of valve calcification in the current study, which is currently a better validated endpoint than 18F-NaF PET.
Conclusion
Among patients with mild to moderate AS, we observed that valvular 18F-NaF uptake, a marker of calcification activity, is predominantly determined by established calcium burden. The results do not support our hypothesis that Lp(a) is associated with valvular 18F-NaF uptake. Nevertheless, due to the unexpected imbalance of competing risk factors in our study population, we cannot rule out that Lp(a) propagates calcification activity in patients with AS with high Lp(a) levels. Our results imply that the established valvular calcific burden is the most important disease driver of AS. Further research evaluating the contribution of Lp(a) at different stages of AS severity is warranted.
Key messages.
What is already known on this subject?
There is substantial evidence that lipoprotein(a) (Lp(a)) is a causal risk factor for incident aortic valve stenosis (AS).
Recent post-hoc analyses suggest that Lp(a) also accelerates AS progression.
What might this study add?
Contrary to our hypothesis, elevated Lp(a) was not associated with a higher degree of calcification activity measured with 18F-sodium fluoride positron emission tomography/CT.
The only driver of calcification activity was valvular Agatston score.
These data suggest that the association between Lp(a) and disease progression may depend on the severity of aortic stenosis as well as other competing risk factors.
How might this impact on clinical practice?
The absence of an effect of Lp(a) on calcification activity in mild to moderate AS may require a shift in focus towards aggressive Lp(a)-lowering in patients with profound Lp(a) elevation prior to the onset of AS.
Acknowledgments
We thank Edwin Poel, Ehsan Hemayat and Meng Fong Lam for their assistance with image acquisition.
Footnotes
Twitter: @MarcDweck, @ArsenaultBenoit, @Zheng_KH
Contributors: KZ, ESGS and MB designed the study. YK and NSN performed patient inclusion. HJV led the PET/CT acquisition. YK, ET and MRD performed image analysis. Statistical analyses were performed by YK and MB. YK wrote the first draft of the manuscript. NSN, JK, HJV, ET, MRD, GAS, ESGS, KHZ and MB critically revised the manuscript. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: YK and ESGS were supported by the Netherlands Heart Foundation CVON 2017-20: generating the best evidence-based pharmaceutical targets for atherosclerosis (GENIUS II). ET is supported by a British Heart Foundation grant (FS/CRTF/20/24086). MRD is supported by the British Heart Foundation (PG/19/40/34422, FS/ICRF/20/26002, FS/14/78/31020, CH/09/002, RG/16/10/32375, RE/18/5/34216).
Competing interests: ESGS has received research grants/support to his institution from Amgen, Sanofi, Resverlogix and Athera, and has served as a consultant for Amgen, Sanofi, Esperion, Novartis and Ionis Pharmaceuticals. All other authors report no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
The study was conducted according to the principles of the International Conference on Harmonization - Good Clinical Practice guidelines. The study protocol was approved by the Ethics Committee of the Amsterdam University Medical Centers, location AMC.
References
- 1. Roth GA, Abate D, Abate KH, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–88. 10.1016/S0140-6736(18)32203-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng KH, Tzolos E, Dweck MR. Pathophysiology of aortic stenosis and future perspectives for medical therapy. Cardiol Clin 2020;38:1–12. 10.1016/j.ccl.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 3. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 4. Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–12. 10.1056/NEJMoa1109034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kamstrup PR, Tybjærg-Hansen A, Nordestgaard BG. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J Am Coll Cardiol 2014;63:470–7. 10.1016/j.jacc.2013.09.038 [DOI] [PubMed] [Google Scholar]
- 6. Arsenault BJ, Boekholdt SM, Dubé M-P, et al. Lipoprotein(a) levels, genotype, and incident aortic valve stenosis: a prospective Mendelian randomization study and replication in a case-control cohort. Circ Cardiovasc Genet 2014;7:304–10. 10.1161/CIRCGENETICS.113.000400 [DOI] [PubMed] [Google Scholar]
- 7. Bajaj A, Rader DJ. Antisense oligonucleotides for atherosclerotic disease. Nat Med 2020;26:471–2. 10.1038/s41591-020-0835-2 [DOI] [PubMed] [Google Scholar]
- 8. Dweck MR, Jenkins WSA, Vesey AT, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging 2014;7:371–8. 10.1161/CIRCIMAGING.113.001508 [DOI] [PubMed] [Google Scholar]
- 9. Zheng KH, Tsimikas S, Pawade T, et al. Lipoprotein(a) and Oxidized Phospholipids Promote Valve Calcification in Patients With Aortic Stenosis. J Am Coll Cardiol 2019;73:2150–62. 10.1016/j.jacc.2019.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972;18:499–502. 10.1093/clinchem/18.6.499 [DOI] [PubMed] [Google Scholar]
- 11. Erqou S, Kaptoge S, et al. , Emerging Risk Factors Collaboration . Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 2009;302:412–23. 10.1001/jama.2009.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pawade TA, Cartlidge TRG, Jenkins WSA, et al. Optimization and reproducibility of aortic valve 18F-Fluoride positron emission tomography in patients with aortic stenosis. Circ Cardiovasc Imaging 2016;9. 10.1161/CIRCIMAGING.116.005131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Massera D, Doris MK, Cadet S, et al. Analytical quantification of aortic valve 18F-sodium fluoride PET uptake. J Nucl Cardiol 2020;27:962–72. 10.1007/s12350-018-01542-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doris MK, Rubeaux M, Pawade T, et al. Motion-corrected imaging of the aortic valve with 18F-NaF PET/CT and PET/MRI: a feasibility study. J Nucl Med 2017;58:1811–4. 10.2967/jnumed.117.194597 [DOI] [PubMed] [Google Scholar]
- 15. Kwiecinski J, Tzolos E, Adamson PD, et al. Coronary 18F-Sodium Fluoride Uptake Predicts Outcomes in Patients With Coronary Artery Disease. J Am Coll Cardiol 2020;75:3061–74. 10.1016/j.jacc.2020.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pawade T, Sheth T, Guzzetti E, et al. Why and How to Measure Aortic Valve Calcification in Patients With Aortic Stenosis. JACC Cardiovasc Imaging 2019;12:1835–48. 10.1016/j.jcmg.2019.01.045 [DOI] [PubMed] [Google Scholar]
- 17. van der Valk FM, Bekkering S, Kroon J, et al. Oxidized phospholipids on Lipoprotein(a) elicit arterial wall inflammation and an inflammatory monocyte response in humans. Circulation 2016;134:611–24. 10.1161/CIRCULATIONAHA.116.020838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu B, Hafiane A, Thanassoulis G, et al. Lipoprotein(a) Induces Human Aortic Valve Interstitial Cell Calcification. JACC Basic Transl Sci 2017;2:358–71. 10.1016/j.jacbts.2017.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Després A-A, Perrot N, Poulin A, et al. Lipoprotein(a), Oxidized Phospholipids, and Aortic Valve Microcalcification Assessed by 18F-Sodium Fluoride Positron Emission Tomography and Computed Tomography. CJC Open 2019;1:131–40. 10.1016/j.cjco.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol 2015;66:561–77. 10.1016/j.jacc.2015.05.066 [DOI] [PubMed] [Google Scholar]
- 21. Capoulade R, Yeang C, Chan KL, et al. Association of Mild to Moderate Aortic Valve Stenosis Progression With Higher Lipoprotein(a) and Oxidized Phospholipid Levels: Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol 2018;3:1212–7. 10.1001/jamacardio.2018.3798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Messika-Zeitoun D, Bielak LF, Peyser PA, et al. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol 2007;27:642–8. 10.1161/01.ATV.0000255952.47980.c2 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen V, Cimadevilla C, Estellat C, et al. Haemodynamic and anatomic progression of aortic stenosis. Heart 2015;101:943–7. 10.1136/heartjnl-2014-307154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2021-319804supp001.pdf (47.5KB, pdf)
Data Availability Statement
No data are available.