Figure 1.
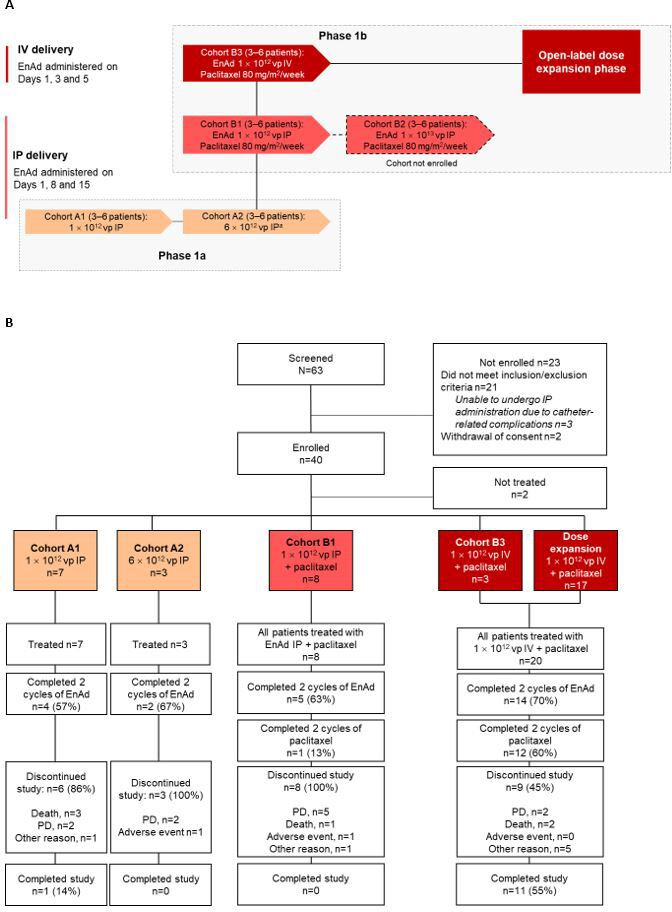
OCTAVE study design (A) and patient disposition (B). (A) IP enadenotucirev monotherapy escalation (phase 1a) was conducted in parallel with the phase 1a combination dose-escalation, starting when the first feasible level of enadenotucirev monotherapy was determined in Phase Ia. In the combination therapy cohorts, paclitaxel was given on days 9, 16 and 23 of each cycle. (B) OCTAVE study patient disposition. aPlanned dose level of 1×1013. EnAd, enadenotucirev; IP, intraperitoneal; IV, intravenous; PD, progressive disease; vp, viral particle.
