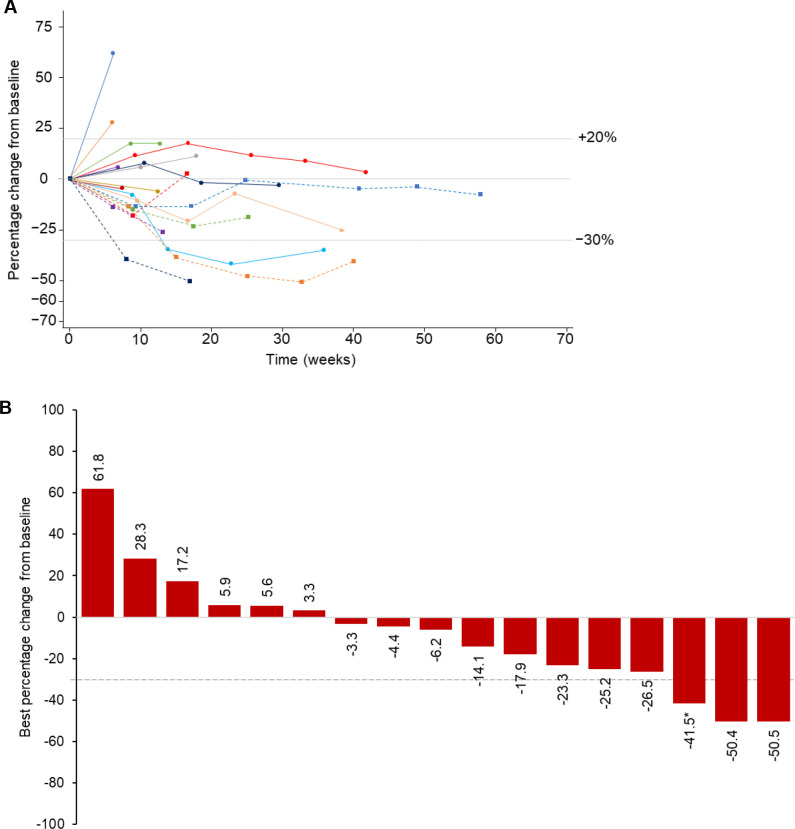
Figure 3.
Change in target lesion burden over time (A) and best change in target lesion burden (B) per Independent assessment (RECIST V.1.1; patients receiving intravenous enadenotucirev plus paclitaxel). Evaluable patients: n=17. (A) Percentage change from baseline in target lesion burden over time in the phase 1b intravenous enadenotucirev plus paclitaxel cohort. Each line represents an individual patient. (B) Best percentage change from baseline in target lesion burden (sum of diameters of target lesions per RECIST V.1.1) according to Independent review. Dashed line indicates 30% decrease in target lesion burden. Each bar represents an individual patient. *Patient achieved PR at one assessment, but then had new lesion progression on the confirmatory scan so response was categorized as PD. PD, progressive disease; PR, partial response.