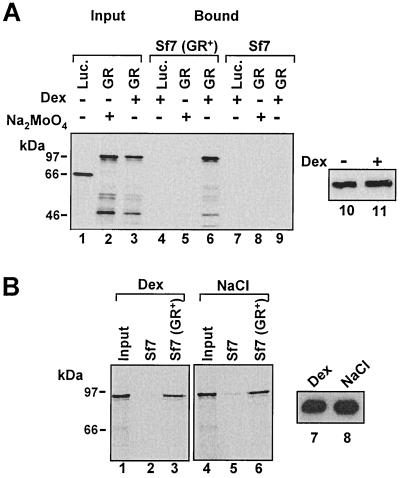
FIG. 1.
Binding of in vitro-translated, 35S-labeled GR to myGR immunoprecipitated from whole-cell extracts is dependent on hsp dissociation. (A) Immunoprecipitates with myc epitope antibody 9E10 from whole-cell extracts prepared from Sf7 cells expressing myGR (lanes 4 to 6) or control cells lacking GR (lanes 7 to 9) were tested for binding to in vitro-translated firefly luciferase (Luc.) or WT GR. Both the cells and in vitro-translated receptors were treated with 10−6 M Dex, as indicated in the figure and described in detail in Materials and Methods. For binding reactions performed in the absence of steroid, association of GR with the chaperone complex was stabilized in cell extracts prior to immunoprecipitation and in the binding assays through the inclusion of 20 mM Na2MoO4 in all buffers as indicated. Specific myGR binding (Bound, lanes 4 to 9) was revealed by SDS-PAGE and fluorography and is compared to 10% of the in vitro-translated proteins added to the binding-assay mixture (Input, lanes 1 to 3). Loading of the GR immunoprecipitates with or without hormone is revealed by Western blotting in lanes 10 and 11. (B) 9E10 immunoprecipitates from Sf7 and Sf7 (GR+) cells were tested for binding to in vitro-translated GR. In lanes 1 to 3, the cells and in vitro-translated receptors were treated with 10−6 M Dex, while in lanes 4 to 6, the immunoprecipitates and in vitro-translated GRs were treated with 0.4 M NaCl to strip the hsp-immunophilin complex from the receptor prior to binding. Binding of in vitro-translated GR is compared to 10% of the input from the in vitro translation. Loading of the GR immunoprecipitates in the binding assay is revealed by Western blot analysis in lanes 7 and 8.