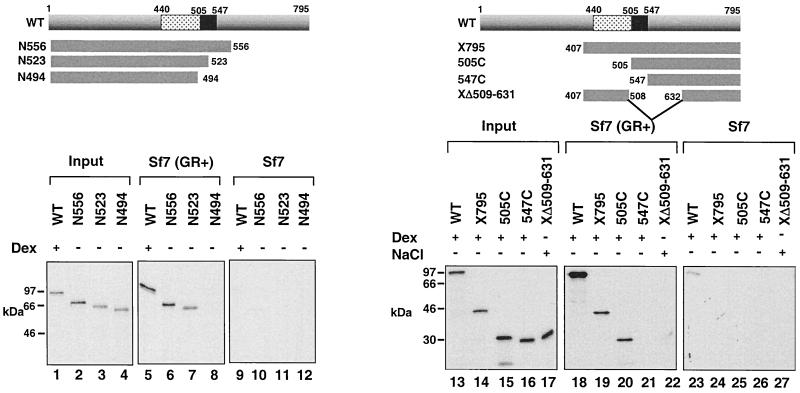
FIG. 2.
Binding of in vitro-translated, 35S-labeled GR to liganded myGR immunoprecipitated from whole-cell extracts is dependent on the receptor hinge region. Immunoprecipitates from whole-cell extracts prepared from Sf7 cells expressing myGR and treated with 10−6 M Dex for 1 h prior to harvesting [Sf7 (GR+), lanes 5 to 8 and 18 to 22] or control Dex-treated cells lacking myGR (Sf7, lanes 9 to 12 and 23 to 27) were tested for binding to in vitro-translated GR derivatives (lanes 5 to 12 and 18 to 27), whose composition is summarized schematically at the top of each panel. In vitro-translated WT GR and GR peptides X795, 505C, and 547C were treated with 10−6 M Dex, while GR peptide XΔ509–631 was treated with 0.4 M NaCl to strip away the chaperone complex prior to incubation with liganded, immunoprecipitated myGR. Dissociation of the in vitro-translated GRs from the hsp complex on Dex and NaCl treatment was confirmed by sedimentation analysis of the receptor over 15 to 30% sucrose gradients (Savory et al., unpublished). Lanes 1 to 4 and 13 to 17 show 10% of the input from the in vitro translations.