Figure 1: Identification and effects of a novel CUL3 variant in a pediatric FHHt patient.
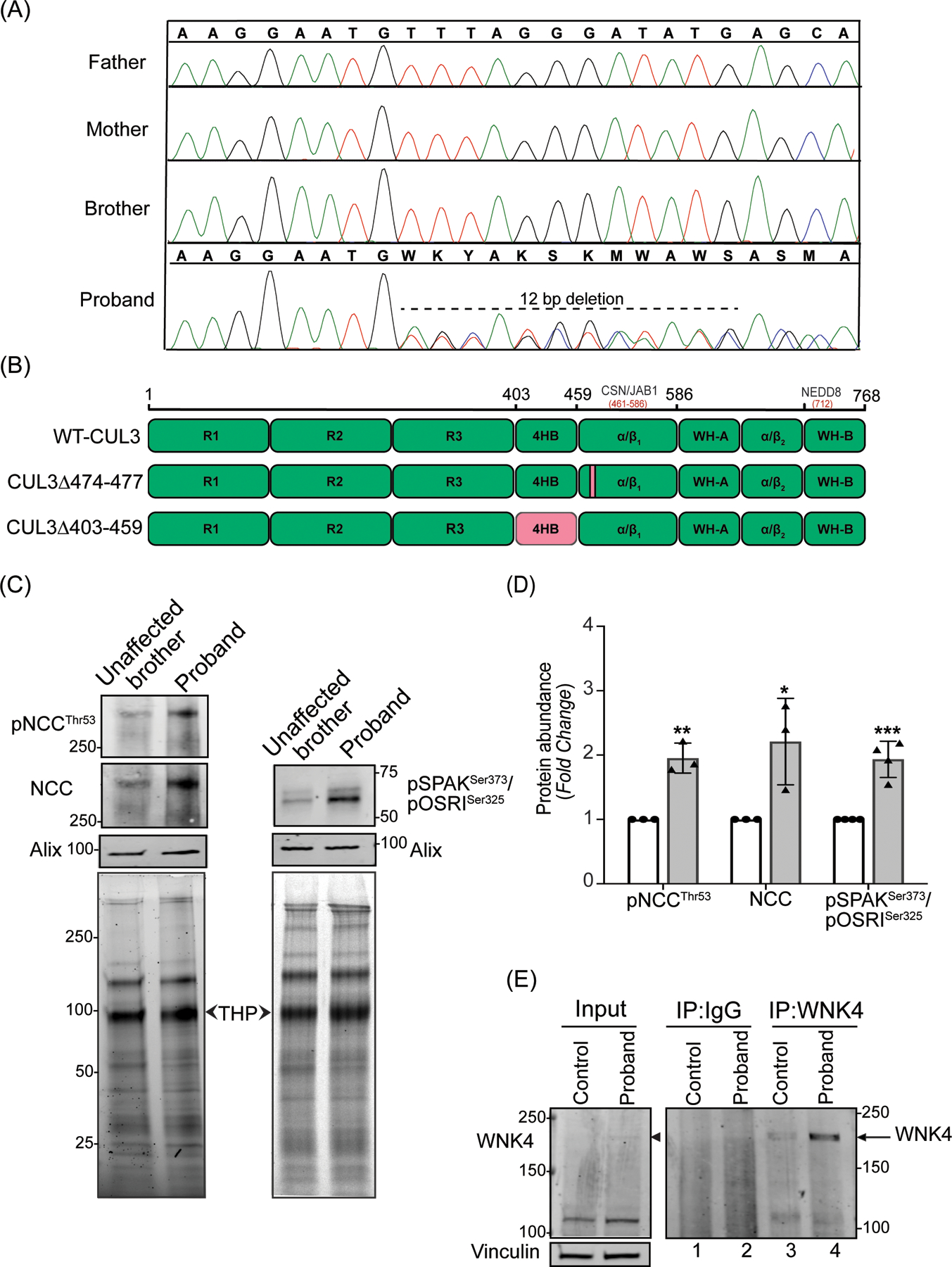
(A) Sanger sequencing chromatograms using genomic DNA from patient (proband) blood samples revealed a de novo heterozygous variant (NM_003590.5: c.1420_1431del12; p. Phe474_Met477del) in the CUL3 gene. CUL3 wild-type genotypes were conserved in the patient’s father, mother, and brother. (B) Domain structure of CUL3 protein shows that the four amino acid deletion (CUL3Δ474–477) in the patient was located within the α/β1 domain of CUL3. This patient’s variant differs from other FHHt-causing CUL3 variants (CUL3Δ403–459), which are located within the 4 helix-bundle (4HB) domain of CUL3. CSN binding site (aa 461–586) is in the α/β1 domain and Nedd8 binding site (K712) is in the WH-B domain of CUL3.
(C) Effects of the CUL3 variant in patient. Immunoblot analysis of uEVs. The patient’s (proband) and his unaffected brother’s urine (first morning void) were processed via ultracentrifugation. Resultant pellets containing uEVs were then treated with dithiothreitol (DTT) to remove Tamm-Horsfall Protein matrices entrapping distal convoluted tubule proteins and lysed in SDS buffer. 10–30 μg of uEVs lysates were separated on 4–15% Tris-Glycine stain-free gels and immunoblotted with antibodies that recognize phosphorylated NCC (pThr53) and total NCC (left panels), and with an antibody that recognizes both phospho SPAK at Ser373 and phospho OSR1 at Ser325 (right panels). Alix was used as a standard marker for uEVs. Imaging of stain-free gels before transfer was used as a loading control. The position of each molecular weight marker in kilodaltons is shown. (D) Quantitative analysis revealed significantly increased phosphorylated NCC, total NCC, and phosphorylated SPAK/OSR1 in the patient’s uEVs when compared to the patient’s unaffected brother’s uEVs. (Samples are technical replicates, error bars denote ± SD, n =3–4, *= P<0.05, **= P<0.01, ***= P<0.001, student t -test). (E) Increased WNK4 abundance in patient’s fibroblasts. Protein lysates from fibroblasts from an unaffected individual (control) and patient were immunoprecipitated and immunoblotted with polyclonal anti-WNK4 antibody. Normal rabbit IgG was used as isotype control antibody to confirm non-specific binding. Immunoblotting of lysates with anti-Vinculin confirmed equal loading of samples.
