Figure 3: The 474–477 deletion alters CUL3 neddylation.
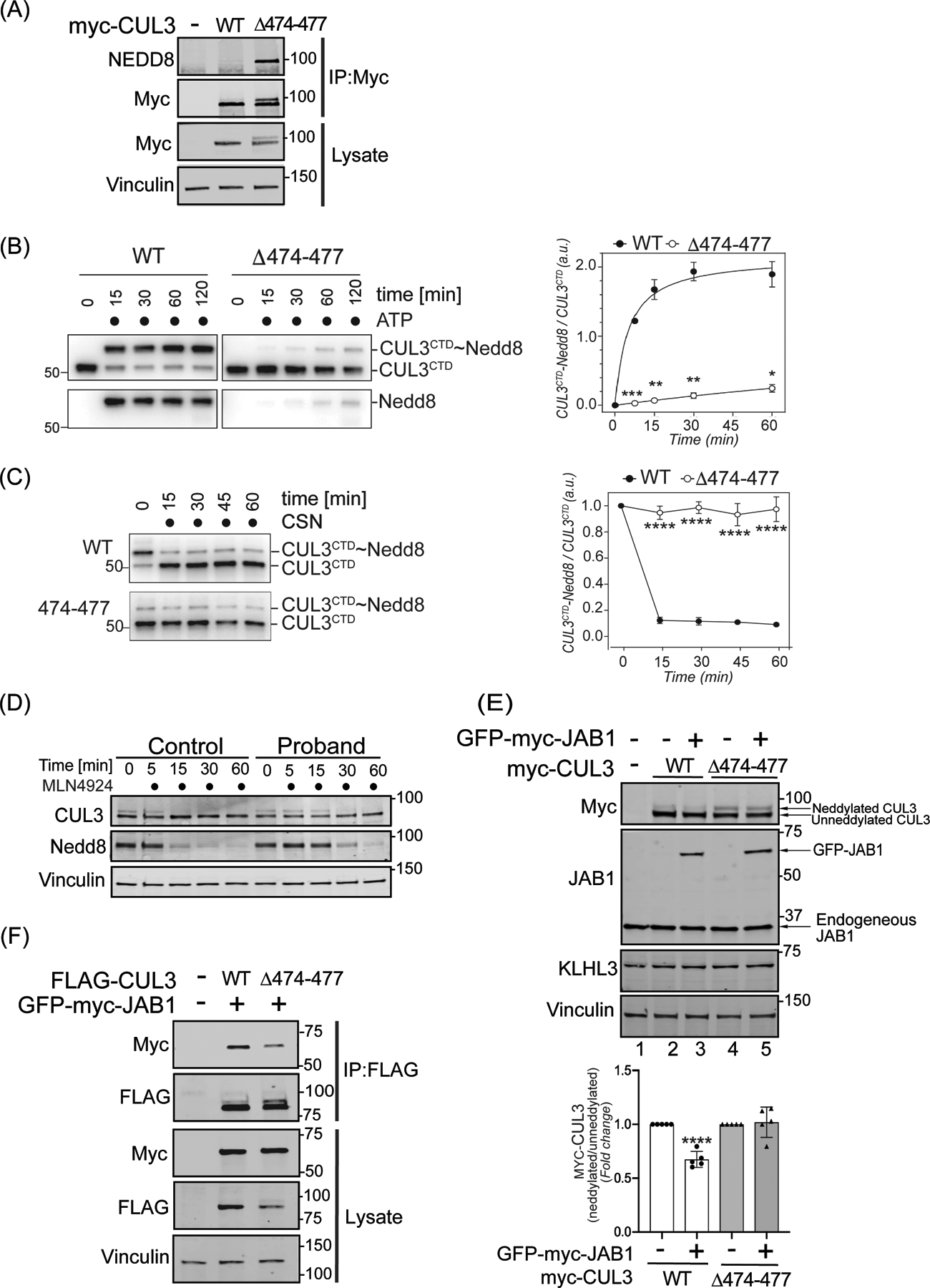
(A) CUL3Δ474–477 is hyperneddylated in HEK293T cells. Whole cell extracts of HEK293T cells overexpressing WT-myc-CUL3 or myc-CUL3Δ474–477 were immunoprecipitated with anti-Myc antibody and immunoblotted with both anti-NEDD8 and anti-Myc antibodies. Immunoblotting with anti-Vinculin was used to confirm equivalent loading of input lysates. (B-C) CUL3Δ474–477 is deficient in reversible modification with NEDD8 in vitro. (B) Left Panel (immunoblots) CUL3/Rbx1 and CUL3Δ474–477/Rbx1 complexes were recombinantly purified using a strategy in which the CUL3 is split into a N-terminal domain (NTD, aa1–309) and C-terminal domain (CTD, aa310–768) and co-expressed with RBX1. Purified Cul3/Rbx1 and Cul3Δ474–477 /Rbx1 complexes were incubated with recombinant NEDD8, NEDD8 E1, and NEDD8 E2 in the absence or presence of ATP for the indicated time periods. Reactions were stopped by addition of urea sample buffer, separated by SDS-PAGE, and subjected to immunoblotting with anti-CUL3 antibodies (detecting the CTD of CUL3) and anti-NEDD8 antibodies. Right Panel (graph) Quantification of neddylation efficiency for the experiment shown in the left panel. The ratio of neddylated CUL3 over unmodified CUL3 is plotted over time (n=5 independently performed in vitro experiments, * = P<0.05, ** = P<0.01, *** = P<0.001, multiple t-test). (C) Left Panel (immunoblots) CUL3/Rbx1 and CUL3Δ474–477 /Rbx1 complexes were maximally neddylated and ATP was quenched by addition of phosphatase. Neddylated CUL3/Rbx1 and CUL3Δ474–477 /Rbx1 were then incubated with recombinant COP9 signalosome (CSN) for the indicated time periods. Reactions were stopped by addition of urea sample buffer, separated by SDS-PAGE, and subjected to immunoblotting with anti-Cul3 antibodies. Right Panel (graph) Quantification of neddylation efficiency for the experiment shown in the left panel. The ratio of neddylated CUL3 over unmodified CUL3 is plotted over time (n=6 independently performed in vitro experiments, **** = P<0.0001, multiple t-test). (D) Neddylation of CUL3 was blocked by the treatment of 500 nM of nedd8-E1 enzyme inhibitor MLN4924 in control and patient fibroblasts. Cells were lysed in RIPA buffer at the indicated time points and lysates were immunoblotted with anti-CUL3 antibody. Vinculin was used to confirm equivalent loading of samples. (E) Upper Panel (immunoblots), JAB1 deneddylation efficiency was evaluated by co-transfecting WT-myc-CUL3 or myc-CUL3Δ474–477 without or with 100 ng GFP-myc-JAB1 in HEK293T cells. Western blot analysis with ant-Myc antibodies showed no obvious change in the deneddylation of CUL3Δ474–477-myc-CUL3, while WT-CUL3 was effectively deneddylated. Anti-JAB1 immunoblot shows both endogenous and over-expressed GFP-JAB1. Equal loading of lysate samples was verified by anti-Vinculin antibody. (Lower Panel) Quantification of deneddylation efficiency of JAB1. The ratio of neddylated/unneddylated CUL3 was calculated in the absence or presence of exogenous JAB1 and shown as fold change. Transfection of 100 ng of JAB1 leads to a significant reduction in the neddylated/unneddylated ratio of WT-CUL3, while neddylated/unneddylated CUL3Δ474–477 remains unaffected (error bars indicate ± SD, n = 5 independent experiments, ****= P<<0.0001, student’s t-test. (F) Reduced interaction of CUL3Δ474–477 with CSN subunit JAB1. HEK293T cells underwent transient co-transfection of WT-FLAG-CUL3 or FLAG-CUL3Δ474–477 along with GFP-myc-JAB1. Immunoprecipitation of lysates with anti-FLAG antibody and subsequent immunoblotting with anti-Myc antibody revealed reduced interaction between JAB1 and CUL3Δ474–477.
