Figure 4: CUL3Δ474–477 affects WNK4 ubiquitination without altering KLHL3 abundance.
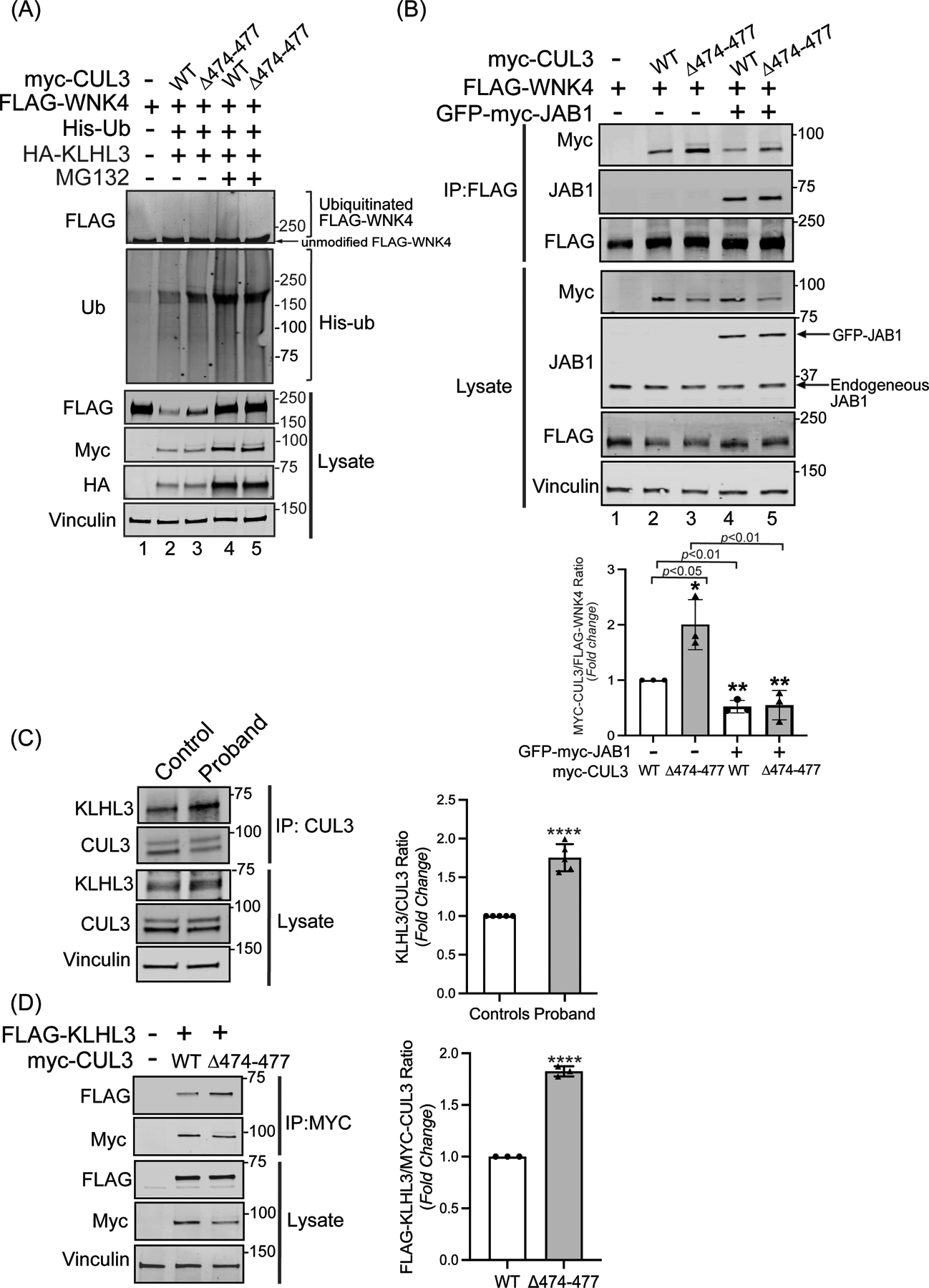
(A) The CUL3Δ474–477 variant leads to reduced ubiquitination of WNK4. HEK293T cells expressing WT-myc-CUL3 or myc-CUL3Δ474–477 were cotransfected with FLAG-WNK4, HA-KLHL3, and His-ubiquitin. Cells were treated with 50 μM MG132 for 5 hrs. before harvesting. Ubiquitin conjugates were purified under denaturing conditions and immunoblotted with an anti-FLAG antibody. Panels labeled ‘Lysates’ indicate samples not subjected to HIS purification and directly immunoblotted with designated antibodies. (B) Upper Panel: Interaction of CUL3Δ474–477 with substrate WNK4. HEK293T cells were co-transfected with WT-myc-CUL3 or myc-CUL3Δ474–477 along with FLAG-WNK4 in the absence or presence of 250 ng of GFP-myc-JAB1. Cells lysates were subjected to immunoprecipitation with anti-FLAG antibody. Immunoblotting of precipitates with anti-Myc antibody revealed interactions of WNK4 with both WT-CUL3 and CUL3Δ474–477, however, the interaction with CUL3Δ474–477 is significantly higher compared to WT-CUL3. Immunoblotting of precipitates with anti-JAB1 antibody also revealed interactions of WNK4 with JAB1 in the presence of WT-CUL3 or CUL3Δ474–477. Immunoblotting of lysates, not subjected to immunoprecipitation, showed the reduced abundance of myc-CUL3Δ474–477 as compared to WT-myc-CUL3. Immunoblotting of lysates with anti-Vinculin antibody confirmed the equal loading of samples. Lower Panel: Quantitative analysis of myc-CUL3 and FLAG-WNK4 bands in IP samples showed significantly higher interaction (shown by increased CUL3/WNK4 ratio) of CUL3Δ474–477-WNK4 compared to WT-CUL3-WNK4 (error bars indicate ± SD, n = 3 independent experiments, *= P<0.05, student’s t-test). The addition of JAB1 significantly reduced the interaction of WNK4 with both WT-myc-CUL3 and MYC-CUL3Δ474–477(error bars indicate ± SD, n = 3 independent experiments, **= P<0.01, student’s t-test) (C) Interaction of KLHL3 with CUL3 is increased in patient’s fibroblasts. Total cellular extracts of patient and control fibroblasts were prepared using RIPA lysis buffer and subsequently immunoprecipitated using an anti-CUL3 antibody. Immunoblotting with anti-KLHL3 antibody (left) and quantitation (right) of KLHL3 and CUL3 bands in IP samples showed significantly higher KLHL3/CUL3 ratio in the proband (samples are technical replicates, error bars indicate ± SD, n = 5 independent experiments, ****= P<0.0001, student’s t-test). Direct immunoblotting of lysates with anti-KLHL3 antibody demonstrated no significant difference in abundance between proband and control fibroblasts. Immunoblotting of lysates with anti-Vinculin antibody showed sample loading. (D) Interaction of KLHL3 with CUL3 was analyzed in HEK293T cells overexpressing FLAG-KLHL3 cotransfected with either WT-myc-CUL3 or myc-CUL3Δ474–477. Immunoprecipitation with anti-Myc antibody and subsequent immunoblotting of lysates with anti-FLAG antibody revealed increased interaction between FLAG-KLHL3 and MYC-CUL3Δ474–477 compared to WT-MYC-CUL3 (upper panels, IP). Quantitation of bands (right) in IP samples showed significant difference (error bars indicate ± SD, n = 3 independent experiments, ****= P<0.0001, student’s t-test). Direct immunoblotting of lysates with anti-FLAG antibody demonstrated no change in FLAG-KLHL3 abundance between WT-myc-CUL3 and myc-CUL3Δ474–477 over-expressed lysates. Vinculin was used to confirm the equivalent loading of the samples.
