Figure 3. Examples of Size Control.
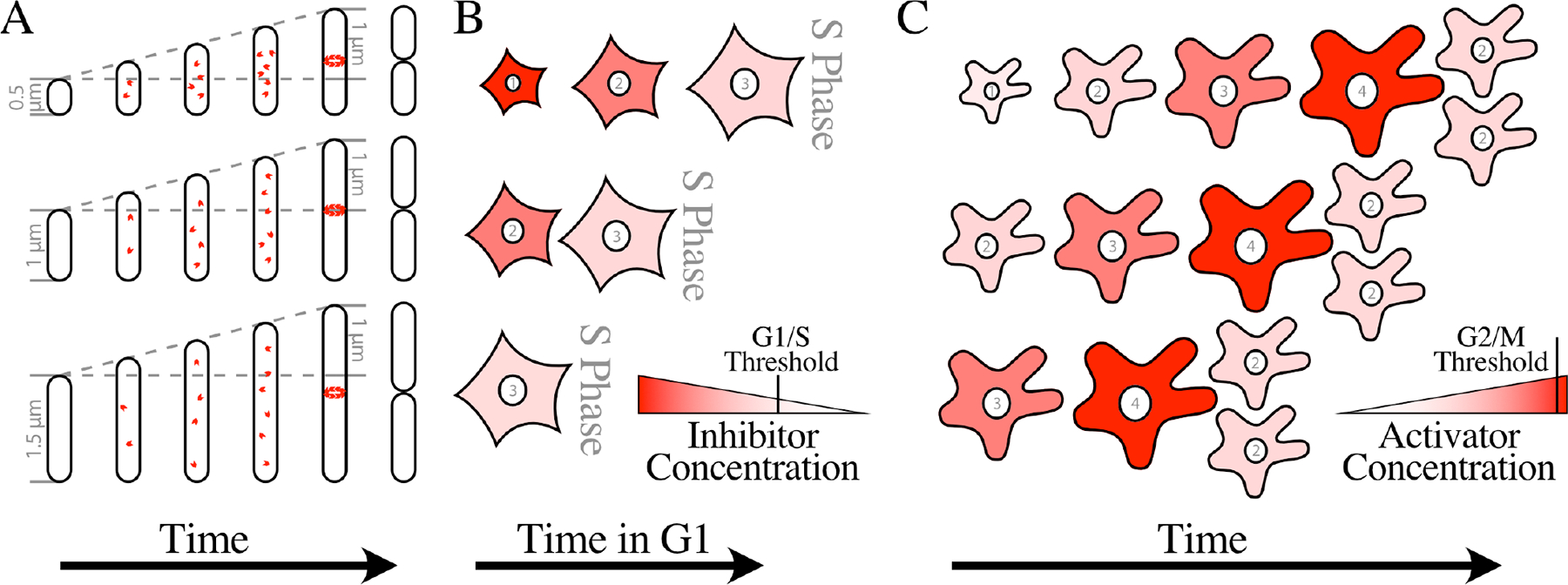
A) A Bacterial Adder. One model for how an adder mechanism could work is by way of a division protein, a protein that must accumulate to a certain number in order for a structure required for division to be formed. FtsZ, the tubulin homolog that forms to contractile ring required for cell division, shown here as red subunits, has been proposed to be such a division protein. If the division protein accumulates proportionally to cell growth, as most proteins do, a fixed amount of growth would be required each cell cycle, creating a adder mechanism. In the simplest division-protein models, the division protein is degraded at division, resetting the system. In this depiction, each cell grows 1 μm, regardless of its birth size. Note that the progeny of small cells are born smaller than the target size of 1 μm, but larger than their parent, moving the average population size towards the target size. A similar model has been proposed for regulation of DNA replication. In that case, a fixed number of DnaA, the replication initiator, are proposed to be required to build an initiation complex at the replication origin.
B) A Diluted-Inhibitor Sizer. In one version of the diluted-inhibitor model, every cell is born with the same amount of an inhibitor, regardless of size. Therefore, smaller cells, depicted here as mammalian fibroblasts, have a higher concentration of inhibitor. The numbers in the nuclei indicate the relative cell sizes, which are roughly equivalent to picoliters, and the intensity of the color indicates the concentration of the inhibitor. As cell grow, they dilute the inhibitor, eventually reaching a critical size that dilutes the inhibitor below a threshold that allows S phase to proceed. Budding yeast, mammalian cells and plants have all been proposed to use a diluted inhibitor to regulate size at the G1/S transition.
C) An Accumulating-Activator Sizer. Accumulating activators are proposed to increase in concentration in proportion to cell size, depicted here in amoeba. The numbers in the nuclei indicate the relative cell sizes, which are roughly equivalent to nanoliters, and the intensity of the color indicates the concentration of the activator. In small cells, the activator concentration is insufficient to drive mitosis, but as cells grow they reach a size at which the activator accumulates to a concentration sufficient to trigger division. Thus all cells divide at about the same size, regardless of their size at birth. Fission yeast, amoeba and Tetrahymena have all been proposed to use an accumulating activator to regulate size at the G2/M transition.
