FIGURE 11.
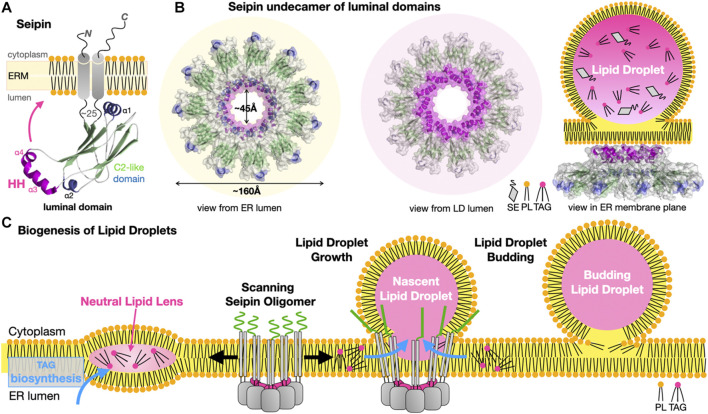
Seipins at the Endoplasmic Reticulum-Lipid Droplet interface. (A) Structure of the Hs/Dm seipin monomers. Two transmembrane helices anchor a luminal domain to the ER. The evolutionarily conserved β-sandwich domain (or C2-like) contains a membrane-anchoring hydrophobic helical (HH) insertion (magenta). (B) Undecamer of Hs-seipin (Dm forms similar dodecamers) shown in transparent surface representation highlighting the luminal HH α-helices involved in ER anchoring. Three views from the ER and LD lumens and, sideways in the ER membrane plane, are shown to emphasize the cup- or funnel- shaped structure and likely mode of membrane association of the seipin oligomers on the ER-luminal side of a nascent LD. Secondary structure elements are colored the same way in (A, B). (C) Model for the biogenesis of lipid droplets from their nucleation to their growth. Oligomers of seipin (grey) scan the ER membrane lateral plane for the presence of neutral lipid lenses. The HH motifs (magenta) acts as a scaffold regulating the surface tension while the N-terminal extensions (green) further stabilize the seipin-nascent LD MCS. The luminal domain of seipin facilitates the lateral transfer of TAGs to the growing LD (PDB 6MLU-6DS5 EMD-9146 EMD-8909).