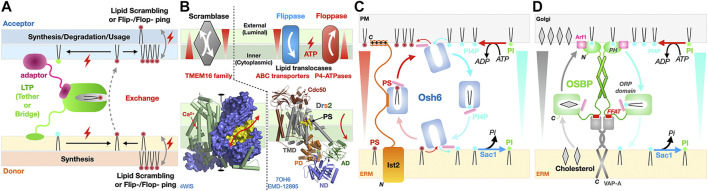
FIGURE 12.
Directionality and energetics of lipid transfer and formation of gradients and membrane asymmetry. (A) General factors determining directionality of lipid transfer at MCS. A hypothetical LTP is depicted here as directly tethered to the donor membrane and attached to the acceptor membrane via an adaptor protein. (B) Scramblases are bi-directional and equilibrate lipid concentrations between leaflets while flippases/floppases require ATP hydrolysis to transport lipids unidirectionally against the gradient to generate membrane asymmetry. Symmetric dimer of the fungal Ca2+ activated-TMEM16 lipid scramblase from Nectria haematococca shown as a solvent accessible surface (blue) to emphasize the presence of a hydrophilic cleft (colored in yellow) running along the side of each monomer and spanning the entire width of the membrane; the other monomer is represented as green cylinders with its two calcium ions positioned the middle plane of the membrane (red spheres) that might assist neutralizing negative charges on the PL during its transfer (PDB 4WIS). Structure of yeast P4-ATPase PS flippase Drs2-Cdc50 chaperone complex with its transmembrane (TMD), nucleotide-binding (ND), phosphorylation (PD) and actuator (AD) domains of the flippase labeled. A molecule of PS bound mid-membrane along the putative translocation path is visible (PDB 7OH6 EMD-12895). (C, D) Lipid counterflow transport by tethered LTPs; (C) PS/PI4P counterflow by Osh6 at ER-to-PM contact sites where Ist2 [only represented on the left half of panel (C)] is the ER-to-PM tether that interacts with the soluble LTP Osh6 to localize its lipid transfer activity at this MCS. (D) Cholesterol/PI4P counterflow by dimers of OSBP at ER-to-Golgi contact sites. PI, phosphatidylinositol; PI4P, phosphatidylinositol 4-monophosphate. Directionality of lipid transfer is indicated by the cyclic colored arrows while gradients of PS, PI4P and cholesterol are indicated by shaded triangles. ATP-consuming biosynthesis provides PI4P while its irreversible hydrolysis into PI by the ER-associated phosphatase Sac1 sets the directionality of these two cycles.