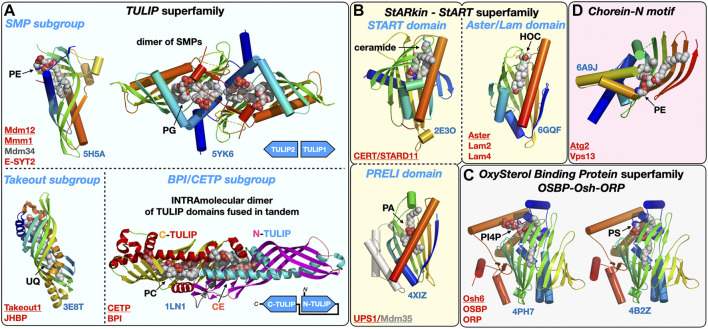
FIGURE 3.
Structural gallery of protein folds and domains observed in lipid-transfer proteins. (A) TULIP protein superfamily representatives within the three subgroups: The SMP domain found in ERMES subunits Mdm12, Mmm1 and Mdm34 and in Extended Synaptotagmins (E-SYT)/Tricalbins (Tcb); the structures of the SMP domains from Mdm12 and Mmm1 (dimer) are shown. The Takeout and BPI/CETP subgroups. (B) StARkin protein superfamily with StART and StART-like domains represented by the ceramide transporter CERT/STARD11 and the sterol-binding Aster/Lam proteins, respectively, and also including the PRELI domain represented by the Ups1/Mdm35 PA shuttle in the mitochondrial intermembrane space. (C) OxySterol Binding Protein superfamily including Osh and ORP proteins, Osh6 is represented bound to the two lipids (PS and PI4P) it counter-exchanges at ER-PM junctions. (D). Chorein-N motif found in proteins Vps13 and Atg2 involved in bulk lipid transfer at several MCSs and the autophagosome, respectively. The motif present in the Atg2 N-terminal fragment crystal structure was observed bound to a PE molecule. Ligands are represented with spheres. Proteins are colored with a rainbow pattern except for the CETP/BPI protein where the two TULIP domains present in tandem are colored differently to highlight the head-to-head intramolecular arrangement of the two TULIP domains. UQ, ubiquinone; CE, cholesteryl-ester; PI4P, phosphatidyl-inositol 4-phosphate; HOC, hydroxycholesterol. Underlined names correspond to the displayed structures (PDB 5H5A-5YK6-3E8T-1LN1-4XIZ-2E3O-6GQF-4PH7-4B2Z-6A9J).