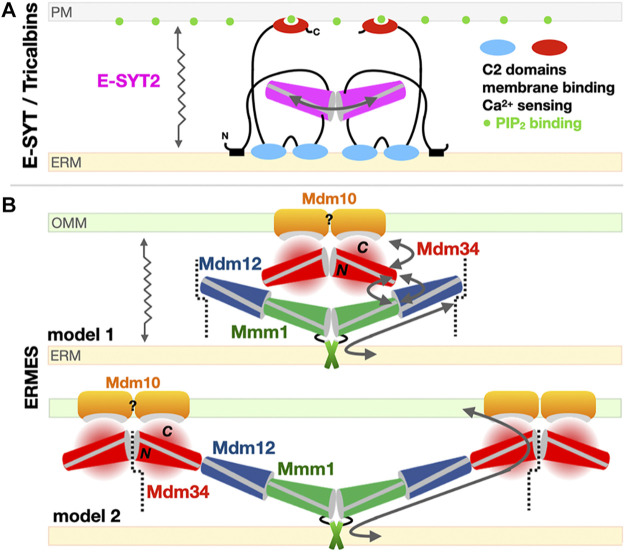
FIGURE 7.
Models for the exchange of phospholipids by SMP domains at contact sites. (A) Tethered shuttle model for SMP-mediated PL exchange by E-SYT2 (yeast Tricalbins) at ER-PM contact sites. E-SYT2 forms dimers via its central SMP domain and is tethered to both membranes (ER and PM) via N-terminal hydrophobic segments and three C-terminal C2 membrane binding domains. The most C-terminal C2 domain binds to PIP2 (PM) and is regulated by cytosolic Ca2+ levels. Given its dimensions a sole dimer of E-SYT SMPs cannot directly bridge one membrane to the other; the flexible linkers are long enough to enable the back-and-forth movement of the dimerized SMPs between the two membranes, alternatively extracting and releasing PLs. (B) Possible “shuttle with handover” versus “lipid conduit”models of assembly of the three SMP domains in ERMES at ER-Mitochondrion contact sites. Based on the Mdm12/Mmm1 SMP tetramer structure and the reported dimeric association state of Mdm34 SMP, two models can be proposed. Mdm10 is shown as a possible dimer while Gem1 has been omitted for simplicity. The dotted line indicates a possible mode of pseudo “infinite” polymerization of whole independent ERMES units supporting the formation of foci. For Mdm34, N and C refer to its N-terminal SMP domain and the uncharacterized and possibly disordered C-terminal region, respectively. The zigzagging arrows indicate that tethered-diffusion of SMP domains (in E-SYT2 and possibly in ERMES) is necessary to lipid transfer by enabling back-and-forth movement of their LTP domains between membranes.