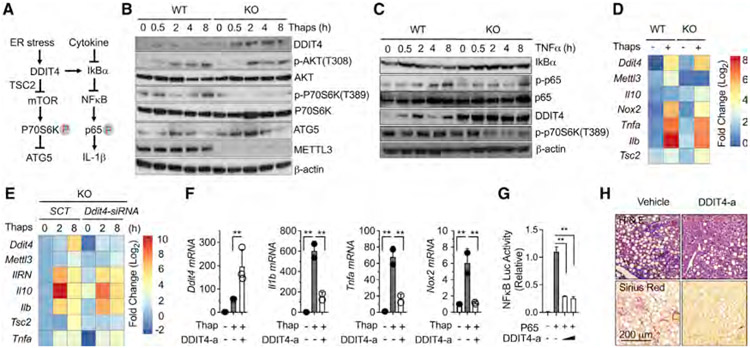
Figure 4. m6A-decorated DDIT4 signatures negatively regulate mTORC1 and NF-κB signaling activation in obesity and NAFLD.
(A) Proposed DDIT4 activity in response to ER stress and inflammatory cytokine stimulation.
(B) Western blot analysis of DDIT4, ATG5, and METTL3 protein levels and the phosphorylation of p70S6K and ATK in response to ER stress stimulation by thap (10 μM) in KO versus WT macrophages.
(C) Western blot analysis of IκBα, NF-κB, and DDIT4 protein levels and the phosphorylation of p65 in response to TNF-α (10 ng/mL) stimulation in KO versus WT macrophages.
(D) The mRNA expression levels of DDIT4 signature genes in response to ER stress stimulation in KO versus WT macrophages.
(E) Restored mRNA expression levels after Ddit4 gene ablation in KO macrophages in response to ER stress stimulation.
(F) Reduction of inflammatory gene expression by a pharmacological DDIT4 activator (DDIT4-a) in response to ER stress stimulation.
(G) Reduction of NF-κB promoter activity by DDIT4-a in the presence of forced p65 subunit expression.
(H) Eight-week-old C57B6J mice were fed with HF-CDAA diet with co-current treatment of DDI4 (1 mg/kg) and vehicle control intraperitoneally (i.p.) every other day for 6 weeks (n = 5). Liver sections stained for H&E and Sirius Red (4 images per mouse). Circline indicates typic infiltrating area. Data represent mean ± SD (n = 4). Two-tailed Student’s t test or two-way ANOVA: *p < 0.05.