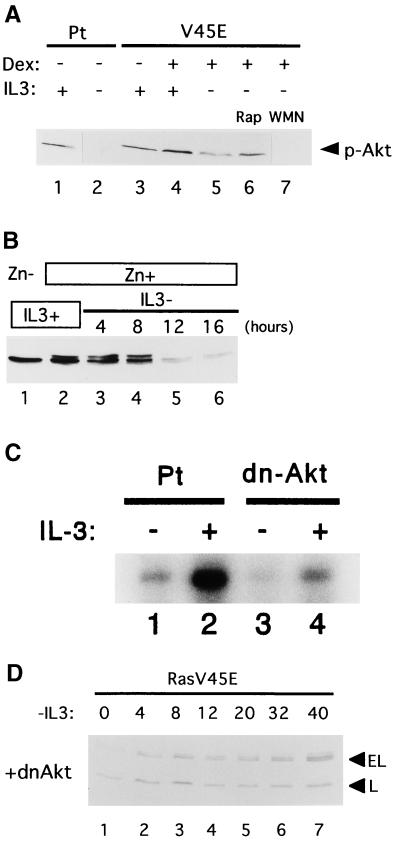
FIG. 6.
(A) Phosphorylation of Akt in Baf-3 cells. Parental (Pt) Baf-3 cells were cultured in the presence (lane 1) or absence (lane 2) of IL-3 for 12 h. Baf-3 cells containing Dexinducible Ras(G12V/V45E) were cultured in IL-3-containing medium without (lane 3) or with (lane 4) 10−7 M Dex for 16 h. The Dex-treated cells were then cultured in IL-3-free, Dex-containing medium for 12 h without wortmannin (WMN) or rapamycin (Rap) (lane 5), with 10 ng/ml rapamycin (lane 6), or with 100 nM wortmannin (lane 7). Akt phosphorylated at Ser473 (top panel) was detected using specific antibodies. (B) Expression of total Akt protein in IL-3-starved Baf-3 cells as detected by immunoblot analysis with antibody recognizing both phosphorylated and nonphosphorylated Akt. Baf-3 cells engineered to express myr-Akt upon addition of Zn were cultured in IL-3-containing medium in the absence (lane 1) or presence (lane 2) of 100 μM Zn for 16 h. The cells were then transferred into IL-3-free medium containing Zn for the indicated times. (C) Akt kinase assay. Parental cells (Pt) (lanes 1 and 2) and cells expressing a dominant-negative form of Akt (dn-Δkt) (lanes 3 and 4) were starved of IL-3 for 2 h (lanes 1 and 3) and then cultured in the presence of IL-3 (20 ng/ml) for 5 min (lanes 2 and 4). The kinase activity of Akt was determined by histone H2B phosphorylation in immunoprecipitates using Akt antibody. (D) Baf-3 cells containing Dex-inducible Ras(G12V/V45E) were retrovirally engineered to express a dominant negative form of Akt (MAA-PKB) (dnΔkt) and then were cultured in IL-3-free, Dex-containing medium for the indicated times.