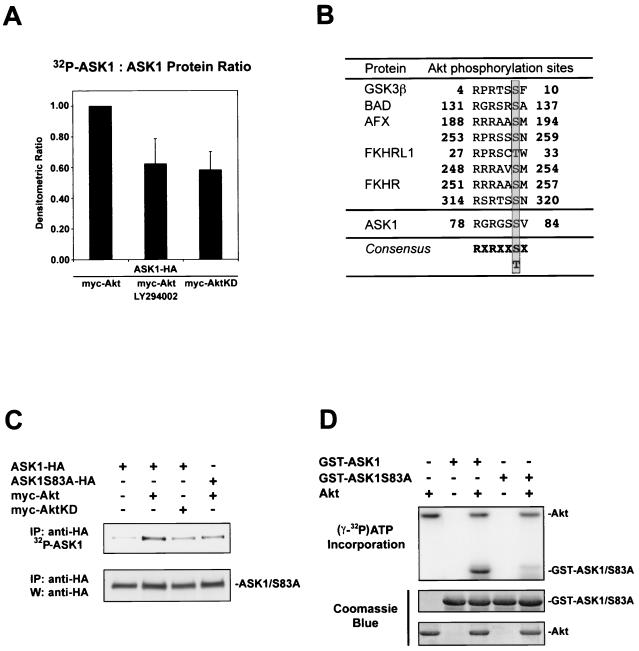
FIG. 1.
Akt phosphorylates ASK1 in cells and in vitro. (A) ASK1 phosphorylation in cells stimulated by serum. 293 cells transiently transfected for 36 h with 1 μg each of the indicated constructs were deprived of phosphate and serum for 1 h, incubated with or without LY294002 (20 μM) for 1 h, and exposed to [32P]orthophosphate and dialyzed serum for 2 h. Immunoprecipitated ASK1 was assessed for 32P labeling (upper panel) and protein levels with anti-HA 3F10 (lower panel). The histogram shows mean densitometric ratios (32P-labeling/protein) plus standard errors of the mean (SEM) from three independent experiments. (B) A consensus Akt phosphorylation site is found in ASK1. Several known Akt substrates are shown. The phosphorylated serine/threonine site is highlighted. (C) Akt phosphorylates ASK1 on serine 83 in IGF-1-stimulated cells. Samples were processed as for panel A except that cells were stimulated for 10 min with 100 ng of IGF-1/ml after [32P]orthophosphate incubation. Dialyzed serum was not present during labeling. Data are representative of three independent experiments. IP, immunoprecipitation; W, Western blot. (D) In vitro phosphorylation of ASK1 by Akt. GST-ASK1 (aa 20 to 118) and GST-ASK1S83A were used as substrates for preactivated recombinant human Akt1 (Akt). [γ-32P]ATP incorporation (upper panel) and protein levels determined by Coomassie blue staining for substrates (middle panel) and Akt (lower panel) are shown. Data are representative of three independent experiments.