Figure 3.
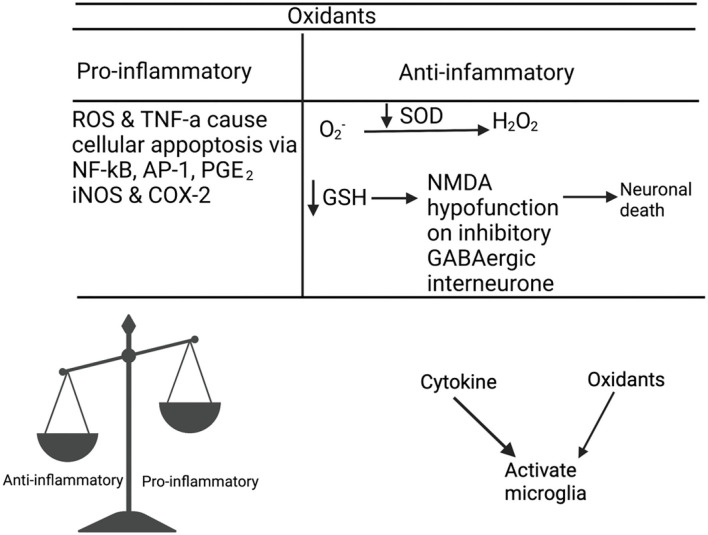
Matsubara and Ziff (59) and Meier et al. (60) reported an interaction between cytokines and oxidants, where they observed the role of TNF-α, IL-1, and IFN-γ in ROS production. Janssen-Heininger et al. (61) Sidoti-de Fraisse et al. (62), and Loukili et al. (63) reported that ROS and TNF-α have a synergistic effect on cell apoptosis via active transcription factors, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and activator protein-1 (AP-1). The increased pro-inflammatory expression, NF-kB, PGE2, iNOS, and COX-2 are unique in patients with schizophrenia compared to bipolar patients or healthy controls (64). Oxidative stress reduces the levels of antioxidants such as glutathione (GSH) (65–69) and glutamate release from microglia (70). Depleted GSH levels cause NMDA hypofunction in inhibitory GABAergic interneurons (71), which fail to mediate inhibitory and excitatory balance of the microcircuitry, resulting in the loss of synapses or neuronal death (72). Incomplete reduction of oxygen generates superoxide anion (), which is converted to hydrogen peroxide (H202) by superoxide dismutase (SOD). SOD is an antioxidant enzyme that prevents oxidative damage from hydroxyl radicals and lipid peroxidation (73). A meta-analyses (74) confirmed that there is a decrease in SOD activity in patients There is an interaction between cytokines, oxidants, and microglia, as TNF-α and NADPH oxidase have been observed to activate microglia in patients with schizophrenia (75–79).