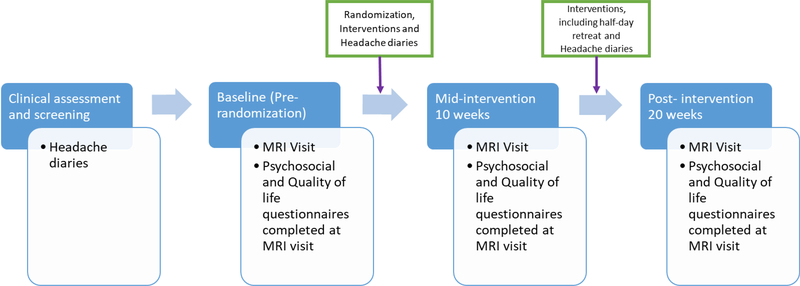
Figure 1: Flow diagram to illustrate the ordering of study activities.
Blue rectangles represent the time periods in the study from pre-baseline when patients were enrolled to post-intervention when patients had completed the last intervention session. Boxes outlined in blue at the bottom highlight activities that take place at the MRI visits at each time point. Boxes with a green outline detail activities that take place between each MRI session. All participants completed headache diaries prior to randomization and the commencement of intervention. Headache diaries were collected over a 28-day period prior to randomization. After the initial 8 weeks of intervention, participants competed their second 28 day headache diary. After the completion of the second 8 weeks of intervention, participants completed their post-intervention 28 day headache diary. All participants completed an MRI visit and the associated questionnaires at baseline prior to the randomization and intervention. The second MRI visit was conducted after the initial 8 weeks of intervention and the third was conducted after the second 8 weeks of intervention.