Abstract
Basophils, which are considered as redundant relatives of mast cells and the rarest granulocytes in peripheral circulation, have been neglected by researchers in the past decades. Previous studies have revealed their vital roles in allergic diseases and parasitic infections. Intriguingly, recent studies even reported that basophils might be associated with cancer development, as activated basophils synthesize and release a variety of cytokines and chemokines in response to cancers. However, it is still subject to debate whether basophils function as tumor-protecting or tumor-promoting components; the answer may depend on the tumor biology and the microenvironment. Herein, we reviewed the role of basophils in cancers, and highlighted some potential and promising therapeutic strategies.
Keywords: Basophil, Cancer, Cytokine, Chemokine, Tumor microenvironment
1. Introduction
Basophils were first discovered by Paul Ehrlich in 1879. They are the least common of the abundant granulocytes, accounting for less than 1% of peripheral leukocytes. Their lifespan in circulation is estimated in days (Karasuyama and Yamanishi, 2014), and they are continually produced and replenished by progenitor cells in the bone marrow. Subsequent studies have identified their typical characteristics of releasing histamine and leukotrienes, as well as their expression of the high-affinity immunoglobulin E (IgE) receptor FcεRI (Schroeder, 2011; Siracusa et al., 2013). In addition, basophils were revealed to produce a strand of cytokines such as interleukin (IL)-4 and IL-13, which are known as key regulators of T helper lymphocyte 2 (Th2) immune responses (Li et al., 1996; Redrup et al., 1998). These findings demonstrated that basophils play crucial roles in allergic disease and immunity to helminths. Moreover, in recent years, novel techniques using basophil-deficient mice and fluorescent protein-labeled basophils allowed us to explore thein vivo functions of basophils and track basophil populations in vivo in diverse immune settings (Obata et al., 2007; Wada et al., 2010; Sullivan et al., 2011). Surprisingly, recent studies have now highlighted previously unrecognized roles of basophils, in which they participate not only in allergic inflammation and parasite infection, but also in cancers (Anthony, 1982; de Monte et al., 2016; Sektioglu et al., 2017; Karasuyama et al., 2018; Webb et al., 2019).
2. Receptors and ligands in basophils
Basophils express a variety of receptors (Fig. 1), and their proliferation, maturity, subsistence, activation, and biological behaviors are closely regulated by relevant ligands in response to various factors.
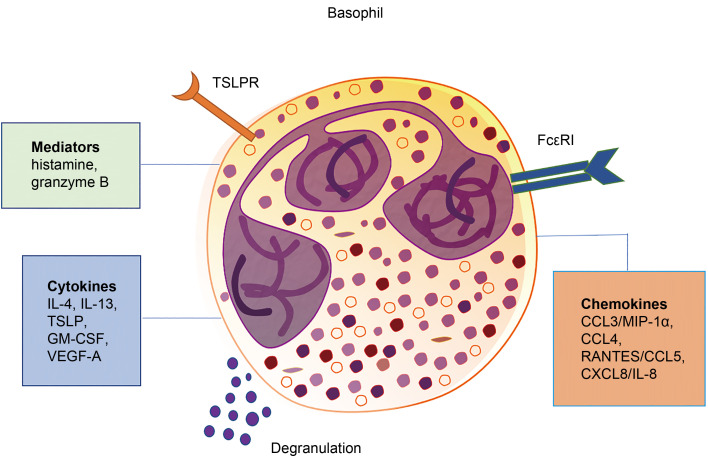
Fig. 1. Surface and secreted molecules of basophils. Basophils express cell surface receptors for immunoglobulin, chemokines, and cytokines, which are important for their development, maturation, homeostasis, and effector functions. They also secrete a variety of molecules, including cytotoxic granule proteins, cytokines, chemokines, and immunoregulatory mediators in response to different stimulations, and play roles in allergic diseases, parasitic infections, and cancers. TSLP: thymic stromal lymphopoietin; TSLPR: TSLP receptor; IL: interleukin; GM-CSF: granulocyte-macrophage colony-stimulating factor; VEGF-A: vascular endothelial growth factor-A; CCL: chemokine (C-C motif) ligand; MIP-1α: macrophage inflammatory protein-1α; RANTES: regulated upon activation normal T cell expressed and secreted factor; CXCL8: chemokine (C-X-C motif) ligand 8.
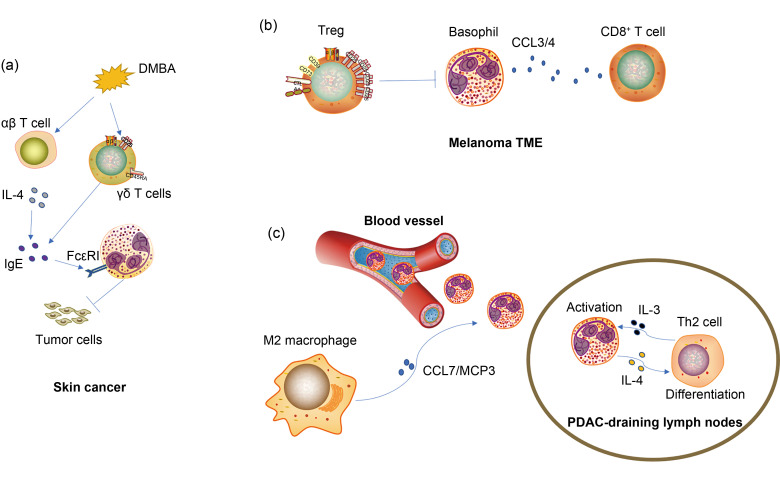
The high-affinity IgE receptor FcεRI is expressed on the surface of basophils (Malveaux et al., 1978). The binding of allergen-specific IgE antibodies to FcεRI when exposed to allergens has been shown to promote allergic disorders (Oda et al., 2019; Engeroff et al., 2020). Crawford et al. (2018) revealed that FcεRI-expressing basophils were closely associated with skin carcinogenesis (Fig. 2a). Their study demonstrated that IgE-effector cells protected against carcinogenesis, and FcεRI-expressing basophils were found to be mainly the IgE-positive cell population in murine skin tumors resulting from exposure to a carcinogen. Moreover, IgE-mediated protection was verified through FcεRI, as mice were more susceptible to the development of tumor in the absence of this high-affinity receptor. This result was consistent with findings of other research (Nigro et al., 2016). Crawford et al. (2018) revealed that the level of FcεRI+ cells was negatively associated with the severity of human squamous-cell carcinomas. These findings revealed that basophils may play vital roles in inhibiting tumor growth through the IgE-FcεRI axis; however, the relevant mechanism remained unclear and further investigation was prompted.
Fig. 2. Mechanisms of recruitment and functions of basophils in the TME. (a) Basophils were recruited and activated in TDLNs under the influence of TME, and regulated the tumor-promoting Th2 immune response in PDAC. The recruitment of basophils into TDLN was closely associated with the secretion of chemokines CCL7/MCP3 by M2 macrophages, and basophil activation was induced by T cell-derived IL-3. (b) Treg depletion in melanoma-bearing mice enhanced tumor infiltration of basophils and CD8+ T cells, leading to the rejection of the tumor. Intratumoral basophils enhanced CD8+ T cell accumulation via the production of chemokines CCL3 and CCL4. (c) Cutaneous exposure to the carcinogen DMBA induced IgE production signaling through FcεRI expressed on basophils, which in turn mediated the protection against carcinogenesis. Moreover, αβ T cell-derived IL-4 was critical for IgE production, while tumor-protective IgE also required γδ T cells. DMBA: 7,12-dimethylbenz[a]anthracene; TDLN: tumor draining lymph node; TME: tumor microenvironment; Th2: T helper lymphocyte 2; PDAC: pancreatic ductal adenocarcinoma; CCL: chemokine (C-C motif) ligand; MCP3: monocyte chemotactic protein 3; IL: interleukin; Treg: regulatory T cell; CD: cluster of differentiation; IgE: immunoglobulin E.
Basophils express the specific IL-3 receptor (IL-3R) throughout their lifespan. IL-3R plays a critical role in the growth, differentiation, survival, and function of basophils (Saito et al., 1988; Mayer et al., 1989; Hagmann et al., 2017; Zellweger et al., 2018). IL-3-elicited basophils support and sustain the development of Th2 immune responses by producing IL-4, a major Th2-type cytokine (Oh et al., 2007; Yoshimoto et al., 2009). Lantz et al. (1998) reported that IL-3, although not indispensable for the production of basophils, contributes to increasing the amount of basophils. Many studies revealed that IL-3 can protect basophils from apoptosis through multiple pathways (Didichenko et al., 2008; Rohner et al., 2018). Apart from regulating allergic inflammation (Gentinetta et al., 2011; Salter et al., 2015) and parasitic infection (Herbst et al., 2012), recent evidence suggested that IL-3 also contributes to allograft fibrosis and chronic rejection by activating basophils (Balam et al., 2019).
Thymic stromal lymphopoietin (TSLP) receptor (TSLPR) was found to be expressed on mast cells and basophils (Siracusa et al., 2011; Varricchi et al., 2018). TSLP could activate basophils through binding to its high-affinity receptor TSLPR in the context of pathological status (Ziegler and Artis, 2010; Siracusa et al., 2011; Varricchi et al., 2018). Siracusa et al. (2011) discovered that TSLP-elicited basophils were different from IL-3-elicited basophils, which indicated that basophils formed a heterogeneous cell population. What is more, TSLP could promote basophil haematopoiesis by acting on bone-marrow resident progenitors and eliciting mature basophil responses in both IL-3-IL-3R-sufficient or -deficient environments.
Suppression of tumorigenicity 2 (ST2), an IL-1 receptor family member that participates in regulating Th2 immune responses, is stably expressed on basophils and activated by its ligand IL-33 (Smithgall et al., 2008). A previous study showed that basophils responded to IL-33 by producing pro-inflammatory cytokines such as IL-4, IL-6, IL-8, IL-13, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Smithgall et al., 2008). Moreover, Schneider et al. (2009) reported that IL-33 could not only activate unprimed basophils, but also elicit basophil expansion in the bone marrow through indirectly promoting the production of hematopoietic growth factors, such as GM-CSF and IL-3. In a model of allergic asthma, IL-33 was able to potentiate the capacity of basophil migration and increase the surface activation markers on basophils during allergen exposure (Salter et al., 2016).
3. Basophil-related cytokines and cancers
A distinct set of cytokines is synthesized and released by basophils (Fig. 1). Among them, the most important ones are IL-4 and IL-13, which are crucial during type 2 immune response, and contribute to different cancers (Nakayama et al., 2017; Shan et al., 2018; Shibata et al., 2018; Galeotti et al., 2019; Yoshikawa et al., 2019).
3.1. IL-4 cytokine
A major cytokine secreted by basophils, IL-4, was found to be important for immune responses ranging from allergy to cancer (Marone et al., 2020b). M2 macrophages have been well known for aggravating tumor progression by enhancing tumor angiogenesis and metastasis (Wang et al., 2018; Zhang et al., 2020). In pancreatic ductal adenocarcinoma (PDAC), basophil-derived IL-4 was found to promote M2 macrophage polarization, thus exerting indirect tumor-promoting function (de Monte et al., 2016). Therein, a high accumulation of basophils was observed in tumor-associated lymph nodes. More importantly, basophil-deficient mice failed to develop tumors, which indicated basophils as crucial contributors to PDAC development. A further investigation showed that T cell-derived IL-3-activated basophils generated and released IL-4, which in turn induced both Th2 and M2 polarization, and consequently contributed to tumor progression (Fig. 2c). Consistently, other groups also revealed that basophil-secreted IL-4 induced M2 skewing (Borriello et al., 2015; Ho et al., 2016).
More importantly, a recent study by He et al. (2021) reported that intratumoral basophils were an independent adverse prognostic factor in gastric cancer, and indicated that IL-4 expression was elevated in the high-basophil group. They also found that the abundance of basophils was closely associated with M2 macrophage infiltration in tumor. The authors concluded that tumor-infiltrating basophils played an essential role in the formation of an immune-evasive tumor microenvironment (TME). In addition, IL-4 was reported to be involved in tumor progression in many malignancies, such as colon cancer, thyroid cancer, lung cancer, breast cancer, and renal cell carcinoma (Shankaranarayanan and Nigam, 2003; Prokopchuk et al., 2005; Falkensammer et al., 2006; Todaro et al., 2006; Zhang et al., 2008; Lin et al., 2019). Strikingly, IL-4 neutralization was found to alter the TME of colon cancer (Ito et al., 2017). Increased cluster of differentiation 8-positive (CD8+) T cell production and granzyme B expression were observed in IL-4 antibody-treated tumors, while angiogenic factors such as vascular endothelial growth factor (VEGF) and epidermal growth factor (EGF) were significantly reduced. In addition, arginase 1 (Arg1), an immunosuppressive factor suppressing T cell activation, as well as M2 macrophage markers, was also found to be downregulated. Interestingly, tumor-associated macrophages (TAMs) isolated from IL-4-depleted tumors failed to inhibit T cell proliferationin vitro, which suggested that IL-4 neutralization impaired the T cell-suppressive ability of TAMs. The study also indicated that the combination of IL-4 antibody and other immunotherapies synergistically inhibited tumor growth and promoted the accumulation of CD4+ and CD8+ T cells in the TME of tumor-bearing mice.
Gocheva et al. (2010) found that cathepsins, as proteases associated with cancer progression, were regulated and activated by IL-4 in TAMs, and thus promoted PDAC invasion and growth in tumor-bearing mice, and concluded that IL-4 could augment TAM tumor-promoting ability, thereby accelerating tumor development. Intriguingly, IL-4 combined with lipopolysaccharide (LPS) was shown to weaken the macrophage-induced invasiveness of PDAC cellsin vitro(Salmiheimo et al., 2016). This effect may be explained by the findings of Wanderley et al. (2018), where LPS could polarize TAM to a tumor-protecting status even in the context of IL-4. Cancer stem cells (CSCs) play an important role in tumor initiation, recurrence, and metastasis (Clara et al., 2020). Colon CSCs were reported to produce IL-4 that was essential to the survival of these cells (di Stefano et al., 2010). Antiapoptotic proteins may be vital for protecting CSCs, as the administration of IL-4 antibody resulted in the downregulated expression of cellular FLICE-like inhibitory protein (cFLIP) and B-cell lymphoma-extra large (Bcl-xL). Moreover, IL-4 blocked enhanced tumor response to standard chemotherapeutic drugs and delayed tumor growth. CSCs showed higher resistance to chemotherapeutic drugs compared with cancer cells, whereas the combination with IL-4 antibody abolished this effect (Gharib et al., 2017). Todaro et al. (2007) suggested that IL-4 protected CSCs from being killed by drug treatment, and concluded that apoptosis-resistant CSCs dictated colon carcinogenesis and therapy refractoriness through the autocrine action of IL-4. Moreover, di Stefano et al. (2010) found that survivin, another apoptosis inhibitor, was increased in colon CSCs regulated by IL-4 via the signal transducer and activator of transcription 6 (STAT6) signaling pathway. To some extent, this was consistent with a different study on breast cancer (Zhang et al., 2008). Similar to CSCs, cancer-initiating cells (CICs) are characterized by the capacity for promoting tumor growth and chemotherapy resistance (Kreso and Dick, 2014; Lima-Fernandes et al., 2019). Volonté et al. (2014) revealed that colorectal CIC-associated IL-4 might be responsible for blocking the proliferation of T cells through targeting IL-4R expressed in T cells, which was dependent on cell-to-cell contact between CICs and T cells. Moreover, IL-4 blocking resulted in the enrichment of CD8+ T cells and enhanced anti-CICs immune responses. These results indicated that the neutralization of IL-4 signaling may shed light on new immunotherapy targeting CICs. Recently, Lin et al. (2019) reported that miR-195-5p, a tumor suppressor, regulated colorectal cancer (CRC) epithelial-mesenchymal transition (EMT), which consequently affected IL-4 expression. More importantly, miR-195-5p-mediated IL-4 secretion resulted in the reduction of M2 macrophage polarization. Costamagna et al. (2020) found that IL-4 administration was capable of prolonging survival and rescuing myogenesis as well as muscle mass in colon cancer-bearing mice. These results proved that IL-4 treatment may improve the quality of life of CRC patients. Overall, basophil-derived IL-4 might participate in regulating CSC survival, angiogenesis, tumor cell proliferation, EMT, and TME.
3.2. IL-13 cytokine
Akin to IL-4, IL-13 is another vital Th2 cytokine responsible for many physiological and pathological activities. Schroeder and Bieneman (2017) revealed that basophils produce IL-13 when cocultured with lung cancer cell line A549. Interestingly, Zhang Y et al. (2018) demonstrated that IL-13 promotes the proliferation and migration of A549 cells. We cannot exclude the possibility that basophil-derived IL-13 might play a role in the growth and invasion of lung cancer. There is accumulating evidence that regulatory T cells (Tregs) contribute to immunosuppressive TME (Sakaguchi et al., 2008). Sharma et al. (2018) found that Tregs could induce basophil activation and promote the release of IL-13. A later study from the same group indicated that Tregs might also mediate tumor immune escape by activating basophils (Das et al., 2020). Liou et al. (2017) reported that IL-13 was expressed in acinar-to-ductal metaplasia and pancreatic intraepithelial neoplasia cells, and differentiated inflammatory macrophages into Ym1+ macrophages in these lesions, which then secreted IL-1 receptor antagonist (IL-1Ra) and chemokine (C-C motif) ligand 2 (CCL2), promoting pancreatic fibrosis and tumor growth. IL-13 was reported to attenuate tumor growth of melanoma and fibrosarcoma-bearing mice possibly via the recruitment of neutrophils and macrophages (Ma et al., 2004). These results indicated that IL-13 played a role in antitumor immunity likely through activating innate immune responses.
Sinha et al. (2005) reported that IL-13 negatively regulated immune surveillance and promoted the development of metastatic breast cancer. Their study revealed that IL-13-deficient mice could generate M1 macrophages, which then played a tumor-protective role by producing nitric oxide (NO) after the surgical resection of a primary tumor. In addition, myeloid-derived suppressor cells (MDSCs) decreased to normal levels after surgery, which reduced the suppression of immune surveillance by CD4+ T cells and CD8+ T cells. However, M1 macrophages alone were not sufficient to eradicate tumors, since IL-4Rα-deficient mice could also generate M1 macrophages but with an elevated MDSC after removal of the primary tumor, and the tumor remained susceptible. These results indicated that effective antitumor immunity required a coordinated interaction of multiple aspects, such as increased NO-producing M1 macrophages and cytotoxic lymphocytes, as well as decreased MSDCs. Aspord et al. (2007) indicated that CD4+ T cell-derived IL-13 promoted the development of breast cancer induced by dendritic cells (DCs), which were instructed by cancer cells in vivo. Moreover, phosphorylated STAT6 was detected in cancer cells, indicating that an IL-13/STAT6 signaling pathway may participate in regulating tumor growth.
Deepak et al. (2007) found that blocking IL-13 activity by delivering decoy IL-13Rα2 resulted in delayed tumor growth and prolonged the survival of Dalton's lymphoma-bearing mice. A later study by the same group showed that neutralization of IL-13 restored and further augmented the production of reactive oxygen intermediate from TAMs in murine Dalton's lymphoma (Deepak et al., 2008). This suggested that a blockade of IL-13 could partially restore macrophage cytotoxicity in Dalton's lymphoma. Future studies should investigate whether basophil-derived IL-13 can modulate tumor growth and the formation of metastasis by acting on other stromal cells in TME in preclinical models and/or in human cancer.
3.3. VEGF-A cytokine
VEGF-A, a component of angiogenesis, is essential for tumor progression and metastasis by delivering oxygen and nutrients (Ferrara and Kerbel, 2005). Several effector cells of inflammation are important sources of angiogenic factors, such as mast cells, macrophages, or basophils (Marone et al., 2016). Studies have shown that activated basophils are a major source of VEGF-A (de Paulis et al., 2006; Marone et al., 2016, 2020a). Thus, basophils might be involved in the complex network of inflammation and tumor angiogenesis, as well as in tumor development. Moreover, supernatants of activated basophils induced an angiogenic response in the chick embryo chorioallantoic membrane, which was inhibited by anti-VEGF-A antibody (de Paulis et al., 2006).
Targeting VEGF-A proved efficient in suppressing tumor growth, metastasis, and vascular leakage in different mouse models including Lewis lung carcinoma (LLC), melanoma, colon cancer, breast tumor, and ovarian carcinoma (Koh et al., 2010). In a recent study, VEGF-A was revealed to contribute to drug resistance, as its silencing inhibited tumor cell proliferation and invasion in dabrafenib-resistant melanoma cells (Caporali et al., 2019). Cheng et al. (2019) found that dying tumor cell-secreted VEGF-A was involved in tumor repopulation, which is a key contributor to tumor recurrence after radiotherapy; the inhibition of VEGF-A or VEGF-A receptors restrained tumor repopulation. This demonstrated that VEGF-A might be a potential target for preventing tumor recurrence after radiotherapy.
In addition, VEGF-A was reported to play a key role in the establishment of an immunosuppressive TME by inhibiting the maturation of DCs, inducing the expansion of MDSCs and promoting the proliferation of Tregs (Terme et al., 2013). Moreover, in a recent study, VEGF-A inhibition abated M2 macrophages and suppressed the revascularization and progression of pancreatic neuroendocrine tumors (Keklikoglou et al., 2018). Studies are urgently needed to determine whether basophil-derived VEGF-A regulates tumor development through interacting with the above immune cells in TME. Collectively, these findings suggested that basophils might be involved in the complex network of tumor angiogenesis by regulating vascular growth factors and their receptors. Future investigations should evaluate the roles of VEGF-A and other angiogenic factors produced by basophils in different tumors.
4. Basophil-related chemokines and cancers
Basophils release several chemokines and express numerous chemokine receptors on their surface (Fig. 1). These chemokines play critical roles in a number of processes, such as leukocyte recruitment and activation, or inflammatory and immune responses (Romagnani, 2002; Russo et al., 2014).
4.1. CCL3/macrophage inflammatory protein-1α (MIP-1α) chemokine
Existing data suggest that CCL3 is an important chemokine augmenting immune cellular infiltration in certain cancers and plays a vital role in antitumor immunity. Allen et al. (2018) indicated that CCL3 augments tumor rejection and enhances CD8+ T cell infiltration in colon cancer. Moreover, they demonstrated that a CCL3-secreting tumor vaccine can effectively reduce the growth of established tumors. Interestingly, evidence by Sektioglu et al. (2017) showed that basophil-derived CCL3 could dampen tumor growth via recruiting CD8+ T cells (Fig. 2b). In their study, basophils infiltrated and were activated in the TME of melanoma in the context of depletion of Treg cells. Moreover, basophil depletion failed to suppress tumor growth, as CD8+ T cells and other immune cells were significantly reduced in the TME. Mechanistically, tumor-derived IL-3, which is pivotal during the lifespan of basophils as discussed above, recruited basophils into the TME, which in turn produced CCL3 to promote the migration and accumulation of CD8+ T cells in the TME, thus leading to the rejection of the tumor.
Furthermore, basophil-derived CCL3 has also been reported to participate in regulating hematological malignancies. Baba et al. (2016) revealed that basophils played an important role in chronic myeloid leukemia (CML) by releasing CCL3. Their study showed that basophil-derived CCL3 impaired the normal hematopoiesis by inhibiting hematopoietic stem/progenitor cell proliferation in the bone marrow. In particular, CCL3-producing basophil-like leukemic cells could induce the expansion of leukemia initiating cells and thus promote the development of CML. Remarkably, blocking C-C motif receptor 5 (CCR5), the receptor of CCL3, significantly prevented CML progression, which indicated that targeting basophil-derived CCL3 might be a promising strategy to improve the therapeutic outcome in CML.
In the LLC mouse model, the treatment with MIP-1α and Propionibacterium acnes, which could induce local inflammatory response, resulted in a reduced tumor burden and prolonged survival in tumor-bearing mice. Moreover, increased myeloid DCs and natural killer (NK) cells, as well as CD8+ T cells, were markedly accumulated in the TME of lung cancer (Nakano et al., 2007). Nakasone et al. (2012) firstly revealed that host-derived MIP-1α was responsible in primary and metastatic melanoma-bearing mice. Both localized tumor growth and lung metastasis were potentiated in the absence of MIP-1α. Specifically, reduced antitumor immunity cells, such as CD4+ T cells, CD8+ T cells, and NK cells, and their cytokine production, including interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), were detected in MIP-1α-deficient mice. Allen et al. (2018) reported that CCL3 suppressed colon tumor growth by modulating innate and adaptive antitumor immunity in the TME. Therein, CCL3 derived from engineered colon cancer cell lines recruited NK cells, which synthetized and released IFN-γ and thus promoted DC accumulation. The CCL3-CCR5 axis was also found to augment the invasive and migratory abilities of tumor cells in esophageal squamous cell carcinoma (ESCC) (Kodama et al., 2020). In the latter study, both TAMs and cancer cell lines were detected to secrete CCL3 and its receptor CCR5, which is observable in cancer cells. A further investigation suggested that CCL3 induced migration and invasion of ESCC by activating the phosphatidylinositol 3 kinase/protein kinase B (PI3K/Akt) and mitogen-activated protein kinase kinase/extracellular regulated protein kinase (MEK/ERK) signaling pathways in tumor cells (Kodama et al., 2020). Altogether, these results indicated that basophil-secreted CCL3 may modulate innate and adaptive immune responses and induce chemokine release, which in turn changes the biological behavior of the tumor. However, whether CCL3 functions as an antitumor or protumor factor is still controversial and needs further elucidation.
4.2. CCL4 chemokine
The inflammatory chemokine CCL4 plays a significant role in the pathogenesis and progression of cancer (Lien et al., 2018). Accumulated evidence suggests that CCL4 is a critical contributor in attracting relevant immune cells to the TME in certain cancers (Spranger et al., 2015; Allen et al., 2018). Liu et al. (2015) found that CCL4 expression was significantly correlated with the expression of CD8 and granzyme B in ESCC, and CCR5 was consistently found to be mainly expressed on CD8+ T cells. Therein, it was indicated that CCL4 might be associated with CD8+ T cell infiltration in TME. Surprisingly, Sektioglu et al. (2017) showed that basophil-derived CCL4 could dampen tumor growth in melanoma (Fig. 2b). In this process, intratumoral basophils produced CCL4 to promote the migration and accumulation of CD8+ T cells in the TME, thus leading to the rejection of the tumor. Similarly, Romero et al. (2020) found that CCL4 was strongly associated with CD8+ T cell infiltration in PDAC. More crucially, Williford et al. (2019) used a tumor stroma-targeting approach to deliver CCL4, which resulted in recruiting DCs and CD8+ T cells in TME and improving the antitumor effect of immunotherapy. These results suggested that basophils might also exert an antitumor immunity function through attracting immune cell accumulation in the TME via secreting CCL4.
Sasaki et al. (2016) reported that CCL4 could mediate interactions between breast cancer cells and intra-bone fibroblasts, which led to breast cancer bone metastasis. Mechanistically, tumor-derived CCL4 induced fibroblasts to express connective tissue growth factor (CTGF)/cellular communication network factor 2 (CCN2) by binding to its specific receptor CCR5, which promoted cancer cell growth and survival in the context of hypoxia. Blocking CCR5 resulted in the reduction of tumor formation and fibroblast numbers. This study indicated that the CCL4-CCR5 axis played an important role in breast cancer bone metastasis, which was considered a promising strategy to suppress tumor metastasis. Lymphatic vessels play vital role in tumor metastasis and immunity (Jiang, 2019). CCL4 was found to promote lymphangiogenesis in oral squamous cell carcinoma (OSCC) (Lien et al., 2018). Therein, CCL4 induced VEGF-C expression in OSCC cells through activating the Janus kinase 2 (JAK2)/STAT3 axis by binding to CCR5 and finally contributing to lymphangiogenesis. Lien et al. (2018) concluded that targeting CCL4 might be a novel efficient strategy to suppress lymphangiogenesis and metastasis in OSCC. Nevertheless, a recent study also found that tumor cell-derived CCL4 plays an antitumor immunity role by recruiting peripheral blood or peritumor regions γδ T-cells into the TME of hepatocellular carcinoma (Zhao et al., 2021). Therefore, these results suggested that basophils and its secretion, CCL4, might be potential target improving cancer immunotherapy particularly in "cold" tumors.
4.3. RANTES/CCL5 chemokine
CCL5, also known as regulated upon activation normal T cell expressed and secreted factor (RANTES), is expressed in various types of immune cells, such as lymphocytes, macrophages, and basophils (Oliver et al., 2010). Nakashima et al. (2014) reported that basophils promoted the recruitment of eosinophils through secreting CCL5. Moreover, the Ito group found that CCL5 induced the accumulation of eosinophils in the TME, thus exerting antitumor immunity (Ito et al., 2017). Consequently, there is a possibility that basophil-derived CCL5 might have tumor-protective effects through acting on eosinophils. Zhang et al. (2015) showed that CCL5 induced breast cancer pulmonary metastasis via promoting CD4+ T cell differentiation into Th2 cells, and the polarized Th2 cells then modulated the metastatic activity of TAMs, which consequently promoted tumor growth and metastasis. Similarly, Zheng et al. (2020) reported that CCL5 modulated breast cancer distant colonization via recruiting macrophages. Moreover, a recent study revealed that CCL5 could induce CCR5-positive macrophage accumulation in residual tumors, which was in turn responsible for collagen deposition and partly resulted in the acceleration of tumor recurrence (Walens et al., 2019).
RANTES/CCL5 was reported to induce immune escape by reducing CD8+ T cell infiltration and mediating interactions between cancer cells and stromal cells, such as macrophages and Treg cells. Chang et al. (2012) found that tumor-derived CCL5 promoted CRC progression through augmenting CD8+ T cell apoptosis induced by transforming growth factor-β (TGF-β) produced by Treg cells in the TME. Moreover, blocking CCR5 or knockdown of CCL5 in tumor-bearing mice resulted in reduced tumor growth, which was associated with lower Treg cell recruitment and the reduction of CD8+ T cell apoptosis. Similarly, Zhang SB et al. (2018) showed that the accumulation of CD8+ T cells in the TME was increased in CCL5-deficient mice. CCL5 has been demonstrated to participate in regulating ovarian cancer initiation and progression through recruiting DCs, promoting the invasion, migration, and differentiation of ovarian CSCs, and mediating angiogenesis in ovarian cancer (Nesbeth et al., 2010; Long et al., 2012; Tang et al., 2016). Interestingly, CCL5 was also reported to induce NK cell accumulation in the TME of melanoma, which subsequently suppressed tumor growth after the depletion of autophagy gene Beclin1 (BECN1) (Mgrditchian et al., 2017). Collectively, these results suggested that whether CCL5 exerted antitumor or protumor immunity was controversial, and this divergence may result from different tumor types and microenvironments. Thus, basophil-derived CCL5 may be responsible for maintaining stem cell-like characteristics altering the infiltration of immune cells, such as NK cells, macrophages, DCs, and CD8+ T cells and inducing CD4+ T cell differentiation, which finally promotes or attenuates tumor progression.
5. Conclusions and challenges
Basophils account for less than 1% of all peripheral blood leukocytes. Despite their small numbers in peripheral blood and tissues, they participate in regulating many disorders, such as allergy, infection, and cancer. To understand the exact role of basophils, technical issues in basophil identification must be first addressed. The current markers to detect basophils are not reliable and are divergent, and therefore, new markers are urgently necessary. Moreover, their functions in allergic disease and parasitic infection have been largely and widely investigated, but there are fewer studies focusing on the role of basophils in the TME. This topic should draw more attention in future studies with great emphasis on the relevant molecular mechanisms.
Immunotherapies targeting the programmed death-1/programmed death-ligand 1 (PD-1/PD-L1) axis have innovated strategies for antitumor immunity and achieved great success in multiple cancers. Tumor cells evade immunological surveillance and avoid being killed by immune cells by expressing PD-L1. Studies have shown that immune cells such as macrophages and neutrophils also express PD-L1 in the TME of cancers, which promotes tumor cell proliferation and suppresses tumor-killing immune cells (Deng et al., 2021; Petty et al., 2021). Intriguingly, it has been reported that basophils express PD-L1 in early lung adenocarcinoma (Lavin et al., 2017). Moreover, tissue resident basophils can induce the polarization of lung macrophages to the M2 subtype (Cohen et al., 2018). However, whether PD-L1+ basophils play the role of anti- or pro-tumorigenesis remains largely unknown and needs thorough investigation to clarify.
The TME is composed of multiple immune cells, such as CD8+ T cells, macrophages, NK cells, Tregs, MDSCs, neutrophils, and eosinophils. Many of these are already known to be involved in regulating tumor initiation and development, either in tumor-promoting or tumor-protective roles. Understanding the role and molecular mechanism of basophils in the TME will be helpful and important to design new targeted drugs that can regulate basophil activity to halt cancer progression. Similar to macrophages and neutrophils that have dual anti- or pro-tumorigenic roles, basophils have also been reported to promote tumor rejection in mice or to be related to reduced survival. Whether this effect depends on tumor type or other factors is still unclear. In addition, targeting basophils, such as their depletion or reprogramming, may trigger uncertain reactions because TME is a complex network composed of immune cells, cancer cells, and other stromal cells. Simultaneous single-cell analysis of the immune landscape of the TME in different tumors can help us to better identify the role of basophils during tumor development. Taken together, many issues need to be urgently addressed, and the answers may be helpful to design novel strategies to improve outcomes for cancer patients, whether combined with current therapy or applied alone.
In summary, the biology of basophils in allergy and infection has been widely investigated. In addition, recent studies have showed that basophils participate in tumor initiation and progression directly or synergistically with other stromal cells by secreting cytokines and chemokines. Further studies should focus on the mechanisms of activation, recruitment, and signaling pathways of basophils in the TME. Single-cell RNA-seq will aid in uncovering the characteristics of basophils in different cancers. The controversy over whether basophils function as tumor-promoting or tumor-protecting cells remains, and thorough investigations are needed to determine their potential as a novel target for future cancer immunotherapies.
Acknowledgments
This work was supported by the Shanghai Sailing Program (No. 21YF1407100), the China Postdoctoral Science Foundation (No. 2021M690037), the National Natural Science Foundation of China (Nos. 82103409 and 81773068), and the National Key R&D Program of China (No. 2019YFC1315902).
Funding Statement
This work was supported by the Shanghai Sailing Program (No. 21YF1407100), the China Postdoctoral Science Foundation (No. 2021M690037), the National Natural Science Foundation of China (Nos. 82103409 and 81773068), and the National Key R&D Program of China (No. 2019YFC1315902).
Author contributions
Jicheng ZHANG, Ning PU, and Wenchuan WU came up with the idea of the manuscript. Jicheng ZHANG, Hanlin YIN, Qiangda CHEN, and Guochao ZHAO wrote the manuscript. Ning PU, Wenhui LOU, and Wenchuan WU revised the manuscript. All authors have read and approved the final manuscript, and therefore, take responsibility for the integrity of the study.
Compliance with ethics guidelines
Jicheng ZHANG, Hanlin YIN, Qiangda CHEN, Guochao ZHAO, Wenhui LOU, Wenchuan WU, and Ning PU declare that they have no conflicts of interests.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Allen F, Bobanga ID, Rauhe P, et al. , 2018. CCL3 augments tumor rejection and enhances CD8+ T cell infiltration through NK and CD103+ dendritic cell recruitment via IFNγ. Oncoimmunology, 7(3): e1393598. 10.1080/2162402X.2017.1393598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony HM, 1982. Blood basophils in lung cancer. Br J Cancer, 45(2): 209-216. 10.1038/bjc.1982.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspord C, Pedroza-Gonzalez A, Gallegos M, et al. , 2007. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med, 204(5): 1037-1047. 10.1084/jem.20061120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T, Tanabe Y, Yoshikawa S, et al. , 2016. MIP-1α/CCL3-expressing basophil-lineage cells drive the leukemic hematopoiesis of chronic myeloid leukemia in mice. Blood, 127(21): 2607-2617. 10.1182/blood-2015-10-673087 [DOI] [PubMed] [Google Scholar]
- Balam S, Schiechl-Brachner G, Buchtler S, et al. , 2019. IL-3 triggers chronic rejection of cardiac allografts by activation of infiltrating basophils. J Immunol, 202(12): 3514-3523. 10.4049/jimmunol.1801269 [DOI] [PubMed] [Google Scholar]
- Borriello F, Longo M, Spinelli R, et al. , 2015. IL-3 synergises with basophil-derived IL-4 and IL-13 to promote the alternative activation of human monocytes. Eur J Immunol, 45(7): 2042-2051. 10.1002/eji.201445303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporali S, Amaro A, Levati L, et al. , 2019. miR-126-3p down-regulation contributes to dabrafenib acquired resistance in melanoma by up-regulating ADAM9 and VEGF-A. J Exp Clin Cancer Res, 38: 272. 10.1186/s13046-019-1238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LY, Lin YC, Mahalingam J, et al. , 2012. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8+ T cells in colon cancer by T-regulatory cells. Cancer Res, 72(5): 1092-1102. 10.1158/0008-5472.CAN-11-2493 [DOI] [PubMed] [Google Scholar]
- Cheng J, He SJ, Wang M, et al. , 2019. The caspase-3/PKCδ/Akt/VEGF-A signaling pathway mediates tumor repopulation during radiotherapy. Clin Cancer Res, 25(12): 3732-3743. 10.1158/1078-0432.CCR-18-3001 [DOI] [PubMed] [Google Scholar]
- Clara JA, Monge C, Yang YZ, et al. , 2020. Targeting signalling pathways and the immune microenvironment of cancer stem cells—a clinical update. Nat Rev Clin Oncol, 17(4): 204-232. 10.1038/s41571-019-0293-2 [DOI] [PubMed] [Google Scholar]
- Cohen M, Giladi A, Gorki AD, et al. , 2018. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell, 175(4): 1031-1044.e18. 10.1016/j.cell.2018.09.009 [DOI] [PubMed] [Google Scholar]
- Costamagna D, Duelen R, Penna F, et al. , 2020. Interleukin-4 administration improves muscle function, adult myogenesis, and lifespan of colon carcinoma-bearing mice. J Cachexia Sarcopenia Muscle, 11(3): 783-801. 10.1002/jcsm.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford G, Hayes MD, Seoane RC, et al. , 2018. Epithelial damage and tissue γδ T cells promote a unique tumor-protective IgE response. Nat Immunol, 19(8): 859-870. 10.1038/s41590-018-0161-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Stephen-Victor E, Bayry J, 2020. Regulatory T cells do not suppress rather activate human basophils by IL-3 and STAT5-dependent mechanisms. Oncoimmunology, 9: 1773193. 10.1080/2162402x.2020.1773193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepak P, Sanjay K, Acharya A, 2007. IL-13 Ralpha2-mediated interleukin-13 neutralization represses in vivo progressive growth of a T-cell lymphoma. J Exp Clin Cancer Res, 26(3): 347-352. [PubMed] [Google Scholar]
- Deepak P, Kumar S, Acharya A, 2008. Interleukin-13 neutralization modulates interleukin-13 induced suppression of reactive oxygen species production in peritoneal macrophages in a murine T-cell lymphoma. Cell Immunol, 251(2): 72-77. 10.1016/j.cellimm.2008.03.005 [DOI] [PubMed] [Google Scholar]
- de Monte L, Wörmann S, Brunetto E, et al. , 2016. Basophil recruitment into tumor-draining lymph nodes correlates with Th2 inflammation and reduced survival in pancreatic cancer patients. Cancer Res, 76(7): 1792-1803. 10.1158/0008-5472.CAN-15-1801-T [DOI] [PubMed] [Google Scholar]
- Deng HJ, Kan AN, Lyu N, et al. , 2021. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer, 9: e002305. 10.1136/jitc-2020-002305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paulis A, Prevete N, Fiorentino I, et al. , 2006. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol, 177(10): 7322-7331. 10.4049/jimmunol.177.10.7322 [DOI] [PubMed] [Google Scholar]
- Didichenko SA, Spiegl N, Brunner T, et al. , 2008. IL-3 induces a Pim1-dependent antiapoptotic pathway in primary human basophils. Blood, 112(10): 3949-3958. 10.1182/blood-2008-04-149419 [DOI] [PubMed] [Google Scholar]
- di Stefano AB, Iovino F, Lombardo Y, et al. , 2010. Survivin is regulated by interleukin-4 in colon cancer stem cells. J Cell Physiol, 225(2): 555-561. 10.1002/jcp.22238 [DOI] [PubMed] [Google Scholar]
- Engeroff P, Caviezel F, Mueller D, et al. , 2020. CD23 provides a noninflammatory pathway for IgE-allergen complexes. J Allergy Clin Immunol, 145(1): 301-311.e4. 10.1016/j.jaci.2019.07.045 [DOI] [PubMed] [Google Scholar]
- Falkensammer C, Jöhrer K, Gander H, et al. , 2006. IL-4 inhibits the TNF-α induced proliferation of renal cell carcinoma (RCC) and cooperates with TNF-α to induce apoptotic and cytokine responses by RCC: implications for antitumor immune responses. Cancer Immunol Immunother, 55(10): 1228-1237. 10.1007/S00262-006-0122-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS, 2005. Angiogenesis as a therapeutic target. Nature, 438(7070): 967-974. 10.1038/nature04483 [DOI] [PubMed] [Google Scholar]
- Galeotti C, Stephen-Victor E, Karnam A, et al. , 2019. Intravenous immunoglobulin induces IL-4 in human basophils by signaling through surface-bound IgE. J Allergy Clin Immunol, 144(2): 524-535.e8. 10.1016/j.jaci.2018.10.064 [DOI] [PubMed] [Google Scholar]
- Gentinetta T, Pecaric-Petkovic T, Wan D, et al. , 2011. Individual IL-3 priming is crucial for consistent in vitro activation of donor basophils in patients with chronic urticaria. J Allergy Clin Immunol, 128(6): 1227-1234.e5. 10.1016/j.jaci.2011.07.021 [DOI] [PubMed] [Google Scholar]
- Gharib AF, Shalaby SM, Raafat N, et al. , 2017. Assessment of neutralizing interleukin-4 effect on CD133 gene expression in colon cancer cell line. Cytokine, 97: 66-72. 10.1016/j.cyto.2017.05.022 [DOI] [PubMed] [Google Scholar]
- Gocheva V, Wang HW, Gadea BB, et al. , 2010. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev, 24(3): 241-255. 10.1101/gad.1874010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann BR, Odermatt A, Kaufmann T, et al. , 2017. Balance between IL-3 and type I interferons and their interrelationship with FasL dictates lifespan and effector functions of human basophils. Clin Exp Allergy, 47(1): 71-84. 10.1111/cea.12850 [DOI] [PubMed] [Google Scholar]
- He XD, Cao YF, Gu Y, et al. , 2021. Clinical outcomes and immune metrics in intratumoral basophil-enriched gastric cancer patients. Ann Surg Oncol, 28: 6439-6450. 10.1245/s10434-021-09815-0 [DOI] [PubMed] [Google Scholar]
- Herbst T, Esser J, Prati M, et al. , 2012. Antibodies and IL-3 support helminth-induced basophil expansion. Proc Natl Acad Sci USA, 109(37): 14954-14959. 10.1073/pnas.1117584109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VW, Hofs E, Elisia I, et al. , 2016. All trans retinoic acid, transforming growth factor β and prostaglandin E2 in mouse plasma synergize with basophil-secreted interleukin-4 to M2 polarize murine macrophages. PLoS ONE, 11(12): e0168072. 10.1371/journal.pone.0168072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito SE, Shirota H, Kasahara Y, et al. , 2017. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol Immunother, 66(11): 1485-1496. 10.1007/s00262-017-2043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, 2019. Lymphatic vasculature in tumor metastasis and immunobiology. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 21(1): 3-11. 10.1631/jzus.B1800633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H, Yamanishi Y, 2014. Basophils have emerged as a key player in immunity. Curr Opin Immunol, 31: 1-7. 10.1016/j.coi.2014.07.004 [DOI] [PubMed] [Google Scholar]
- Karasuyama H, Miyake K, Yoshikawa S, et al. , 2018. Multifaceted roles of basophils in health and disease. J Allergy Clin Immunol, 142(2): 370-380. 10.1016/j.jaci.2017.10.042 [DOI] [PubMed] [Google Scholar]
- Keklikoglou I, Kadioglu E, Bissinger S, et al. , 2018. Periostin limits tumor response to VEGFA inhibition. Cell Rep, 22(10): 2530-2540. 10.1016/j.celrep.2018.02.035 [DOI] [PubMed] [Google Scholar]
- Kodama T, Koma YI, Arai N, et al. , 2020. CCL3-CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Invest, 100(9): 1140-1157. 10.1038/s41374-020-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh YJ, Kim HZ, Hwang SI, et al. , 2010. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell, 18(2): 171-184. 10.1016/j.ccr.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Kreso A, Dick JE, 2014. Evolution of the cancer stem cell model. Cell Stem Cell, 14(3): 275-291. 10.1016/j.stem.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Lantz CS, Boesiger J, Song CH, et al. , 1998. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature, 392(6671): 90-93. 10.1038/32190 [DOI] [PubMed] [Google Scholar]
- Lavin Y, Kobayashi S, Leader A, et al. , 2017. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell, 169(4): 750-765.e17. 10.1016/j.cell.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Sim TC, Alam R, 1996. IL-13 released by and localized in human basophils. J Immunol, 156(12): 4833-4838. [PubMed] [Google Scholar]
- Lien MY, Tsai HC, Chang AC, et al. , 2018. Chemokine CCL4 induces vascular endothelial growth factor C expression and lymphangiogenesis by miR-195-3p in oral squamous cell carcinoma. Front Immunol, 9: 412. 10.3389/fimmu.2018.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Fernandes E, Murison A, da Silva Medina T, et al. , 2019. Targeting bivalency de-represses Indian Hedgehog and inhibits self-renewal of colorectal cancer-initiating cells. Nat Commun, 10: 1436. 10.1038/s41467-019-09309-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XB, Wang SY, Sun M, et al. , 2019. miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. J Hematol Oncol, 12: 20. 10.1186/s13045-019-0708-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liou GY, Bastea L, Fleming A, et al. , 2017. The presence of interleukin-13 at pancreatic ADM/PanIN lesions alters macrophage populations and mediates pancreatic tumorigenesis. Cell Rep, 19(7): 1322-1333. 10.1016/j.celrep.2017.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JY, Li F, Wang LP, et al. , 2015. CTL- vs Treg lymphocyte-attracting chemokines, CCL4 and CCL20, are strong reciprocal predictive markers for survival of patients with oesophageal squamous cell carcinoma. Br J Cancer, 113(5): 747-755. 10.1038/bjc.2015.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long HX, Xie RK, Xiang T, et al. , 2012. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells, 30(10): 2309-2319. 10.1002/stem.1194 [DOI] [PubMed] [Google Scholar]
- Ma HL, Whitters MJ, Jacobson BA, et al. , 2004. Tumor cells secreting IL-13 but not IL-13Rα2 fusion protein have reduced tumorigenicity in vivo. Int Immunol, 16(7): 1009-1017. 10.1093/intimm/dxh105 [DOI] [PubMed] [Google Scholar]
- Malveaux FJ, Conroy MC, Adkinson NF, et al. , 1978. IgE receptors on human basophils. Relationship to serum IgE concentration. J Clin Invest, 62(1): 176-181. 10.1172/JCI109103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone G, Varricchi G, Loffredo S, et al. , 2016. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur J Pharmacol, 778: 146-151. 10.1016/j.ejphar.2015.03.088 [DOI] [PubMed] [Google Scholar]
- Marone G, Gambardella AR, Mattei F, et al. , 2020a. Basophils in tumor microenvironment and surroundings. Adv Exp Med Biol, 1224: 21-34. 10.1007/978-3-030-35723-8_2 [DOI] [PubMed] [Google Scholar]
- Marone G, Schroeder JT, Mattei F, et al. , 2020b. Is there a role for basophils in cancer? Front Immunol, 11: 2103. 10.3389/fimmu.2020.02103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer P, Valent P, Schmidt G, et al. , 1989. The in vivo effects of recombinant human interleukin-3: demonstration of basophil differentiation factor, histamine-producing activity, and priming of GM-CSF-responsive progenitors in nonhuman primates. Blood, 74(2): 613-621. 10.1182/blood.V74.2.613.613 [DOI] [PubMed] [Google Scholar]
- Mgrditchian T, Arakelian T, Paggetti J, et al. , 2017. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc Natl Acad Sci USA, 114(44): E9271-E9279. 10.1073/pnas.1703921114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Yoneyama H, Ueha S, et al. , 2007. Intravenous administration of MIP-1α with intra-tumor injection of P. acnes shows potent anti-tumor effect. Int Immunopharmacol, 7(6): 845-857. 10.1016/j.intimp.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Nakashima C, Otsuka A, Kitoh A, et al. , 2014. Basophils regulate the recruitment of eosinophils in a murine model of irritant contact dermatitis. J Allergy Clin Immunol, 134(1): 100-107. 10.1016/j.jaci.2014.02.026 [DOI] [PubMed] [Google Scholar]
- Nakasone Y, Fujimoto M, Matsushita T, et al. , 2012. Host-derived MCP-1 and MIP-1α regulate protective anti-tumor immunity to localized and metastatic B16 melanoma. Am J Pathol, 180(1): 365-374. 10.1016/j.ajpath.2011.09.005 [DOI] [PubMed] [Google Scholar]
- Nakayama T, Hirahara K, Onodera A, et al. , 2017. Th2 cells in health and disease. Annu Rev Immunol, 35: 53-84. https://doi.org/10.1146/annurev-immunol-051116-052350 [DOI] [PubMed] [Google Scholar]
- Nesbeth YC, Martinez DG, Toraya S, et al. , 2010. CD4+ T cells elicit host immune responses to MHC class II- ovarian cancer through CCL5 secretion and CD40-mediated licensing of dendritic cells. J Immunol, 184(10): 5654-5662. 10.4049/jimmunol.0903247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro EA, Brini AT, Yenagi VA, et al. , 2016. Cutting edge: IgE plays an active role in tumor immunosurveillance in mice. J Immunol, 197(7): 2583-2588. 10.4049/jimmunol.1601026 [DOI] [PubMed] [Google Scholar]
- Obata K, Mukai K, Tsujimura Y, et al. , 2007. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood, 110(3): 913-920. 10.1182/blood-2007-01-068718 [DOI] [PubMed] [Google Scholar]
- Oda Y, Fukunaga A, Washio K, et al. , 2019. Low responsiveness of basophils via FcεRI reflects disease activity in chronic spontaneous urticaria. J Allergy Clin Immunol Pract, 7(8): 2835-2844.e7. 10.1016/j.jaip.2019.05.020 [DOI] [PubMed] [Google Scholar]
- Oh K, Shen T, le Gros G, et al. , 2007. Induction of Th2 type immunity in a mouse system reveals a novel immunoregulatory role of basophils. Blood, 109(7): 2921-2927. 10.1182/blood-2006-07-037739 [DOI] [PubMed] [Google Scholar]
- Oliver JM, Tarleton CA, Gilmartin L, et al. , 2010. Reduced FcεRI-mediated release of asthma-promoting cytokines and chemokines from human basophils during omalizumab therapy. Int Arch Allergy Immunol, 151(4): 275-284. 10.1159/000250436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty AJ, Dai R, Lapalombella R, et al. , 2021. Hedgehog-induced PD-L1 on tumor-associated macrophages is critical for suppression of tumor-infiltrating CD8+ T cell function. JCI Insight, 6(6): e146707. 10.1172/jci.insight.146707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokopchuk O, Liu Y, Henne-Bruns D, et al. , 2005. Interleukin-4 enhances proliferation of human pancreatic cancer cells: evidence for autocrine and paracrine actions. Br J Cancer, 92(5): 921-928. 10.1038/sj.bjc.6602416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redrup AC, Howard BP, Macglashan DW, et al. , 1998. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol, 160(4): 1957-1964. [PubMed] [Google Scholar]
- Rohner L, Reinhart R, Hagmann B, et al. , 2018. FcɛRI cross-linking and IL-3 protect human basophils from intrinsic apoptotic stress. J Allergy Clin Immunol, 142(5): 1647-1650.e3. 10.1016/j.jaci.2018.06.040 [DOI] [PubMed] [Google Scholar]
- Romagnani S, 2002. Cytokines and chemoattractants in allergic inflammation. Mol Immunol, 38(12-13): 881-885. 10.1016/S0161-5890(02)00013-5 [DOI] [PubMed] [Google Scholar]
- Romero JM, Grünwald B, Jang GH, et al. , 2020. A four-chemokine signature is associated with a T-cell-inflamed phenotype in primary and metastatic pancreatic cancer. Clin Cancer Res, 26(8): 1997-2010. 10.1158/1078-0432.Ccr-19-2803 [DOI] [PubMed] [Google Scholar]
- Russo RC, Garcia CC, Teixeira MM, et al. , 2014. The CXCl8/IL-8 chemokine family and its receptors in inflammatory diseases. Expert Rev Clin Immunol, 10(5): 593-619. 10.1586/1744666X.2014.894886 [DOI] [PubMed] [Google Scholar]
- Saito H, Hatake K, Dvorak AM, et al. , 1988. Selective differentiation and proliferation of hematopoietic cells induced by recombinant human interleukins. Proc Natl Acad Sci USA, 85(7): 2288-2292. 10.1073/pnas.85.7.2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, et al. , 2008. Regulatory T cells and immune tolerance. Cell, 133(5): 775-787. 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Salmiheimo ANE, Mustonen HK, Vainionpää SAA, et al. , 2016. Increasing the inflammatory competence of macrophages with IL-6 or with combination of IL-4 and LPS restrains the invasiveness of pancreatic cancer cells. J Cancer, 7(1): 42-49. 10.7150/jca.12923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter BM, Oliveria JP, Nusca G, et al. , 2015. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J Allergy Clin Immunol, 136(6): 1636-1644. 10.1016/j.jaci.2015.03.039 [DOI] [PubMed] [Google Scholar]
- Salter BM, Oliveria JP, Nusca G, et al. , 2016. IL-25 and IL-33 induce type 2 inflammation in basophils from subjects with allergic asthma. Respir Res, 17: 5. 10.1186/s12931-016-0321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Baba T, Nishimura T, et al. , 2016. Essential roles of the interaction between cancer cell-derived chemokine, CCL4, and intra-bone CCR5-expressing fibroblasts in breast cancer bone metastasis. Cancer Lett, 378(1): 23-32. 10.1016/j.canlet.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Schneider E, Petit-Bertron AF, Bricard R, et al. , 2009. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J Immunol, 183(6): 3591-3597. 10.4049/jimmunol.0900328 [DOI] [PubMed] [Google Scholar]
- Schroeder JT, 2011. Basophils: emerging roles in the pathogenesis of allergic disease. Immunol Rev, 242(1): 144-160. 10.1111/j.1600-065X.2011.01023.x [DOI] [PubMed] [Google Scholar]
- Schroeder JT, Bieneman AP, 2017. Activation of human basophils by A549 lung epithelial cells reveals a novel IgE-dependent response independent of allergen. J Immunol, 199(3): 855-865. 10.4049/jimmunol.1700055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sektioglu IM, Carretero R, Bulbuc N, et al. , 2017. Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells. Cancer Res, 77(2): 291-302. 10.1158/0008-5472.CAN-16-0993 [DOI] [PubMed] [Google Scholar]
- Shan MM, Carrillo J, Yeste A, et al. , 2018. Secreted IgD amplifies humoral T helper 2 cell responses by binding basophils via galectin-9 and CD44. Immunity, 49(4): 709-724.e8. 10.1016/j.immuni.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaranarayanan P, Nigam S, 2003. IL-4 induces apoptosis in A549 lung adenocarcinoma cells: evidence for the pivotal role of 15-hydroxyeicosatetraenoic acid binding to activated peroxisome proliferator-activated receptor γ transcription factor. J Immunol, 170(2): 887-894. 10.4049/jimmunol.170.2.887 [DOI] [PubMed] [Google Scholar]
- Sharma M, Das M, Stephen-Victor E, et al. , 2018. Regulatory T cells induce activation rather than suppression of human basophils. Sci Immunol, 3(23): eaan0829. 10.1126/sciimmunol.aan0829 [DOI] [PubMed] [Google Scholar]
- Shibata S, Miyake K, Tateishi T, et al. , 2018. Basophils trigger emphysema development in a murine model of COPD through IL-4-mediated generation of MMP-12-producing macrophages. Proc Natl Acad Sci USA, 115(51): 13057-13062. 10.1073/pnas.1813927115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Ostrand-Rosenberg S, 2005. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res, 65(24): 11743-11751. 10.1158/0008-5472.CAN-05-0045 [DOI] [PubMed] [Google Scholar]
- Siracusa MC, Saenz SA, Hill DA, et al. , 2011. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature, 477(7363): 229-233. 10.1038/nature10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siracusa MC, Kim BS, Spergel JM, et al. , 2013. Basophils and allergic inflammation. J Allergy Clin Immunol, 132(4): 789-801. 10.1016/j.jaci.2013.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithgall MD, Comeau MR, Yoon BRP, et al. , 2008. IL-33 amplifies both Th1-and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol, 20(8): 1019-1030. 10.1093/intimm/dxn060 [DOI] [PubMed] [Google Scholar]
- Spranger S, Bao RY, Gajewski TF, 2015. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature, 523(7559): 231-235. 10.1038/nature14404 [DOI] [PubMed] [Google Scholar]
- Sullivan BM, Liang HE, Bando JK, et al. , 2011. Genetic analysis of basophil function in vivo. Nat Immunol, 12(6): 527-535. 10.1038/ni.2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Xiang T, Huang S, et al. , 2016. Ovarian cancer stem-like cells differentiate into endothelial cells and participate in tumor angiogenesis through autocrine CCL5 signaling. Cancer Lett, 376(1): 137-147. 10.1016/j.canlet.2016.03.034 [DOI] [PubMed] [Google Scholar]
- Terme M, Tartour E, Taieb J, 2013. VEGFA/VEGFR2-targeted therapies prevent the VEGFA-induced proliferation of regulatory T cells in cancer. Oncoimmunology, 2(8): e25156. 10.4161/onci.25156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro M, Zerilli M, Ricci-Vitiani L, et al. , 2006. Autocrine production of interleukin-4 and interleukin-10 is required for survival and growth of thyroid cancer cells. Cancer Res, 66(3): 1491-1499. 10.1158/0008-5472.CAN-05-2514 [DOI] [PubMed] [Google Scholar]
- Todaro M, Alea MP, di Stefano AB, et al. , 2007. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell, 1(4): 389-402. 10.1016/j.stem.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Varricchi G, Pecoraro A, Marone G, et al. , 2018. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front Immunol, 9: 1595. 10.3389/fimmu.2018.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonté A, di Tomaso T, Spinelli M, et al. , 2014. Cancer-initiating cells from colorectal cancer patients escape from T cell-mediated immunosurveillance in vitro through membrane-bound IL-4. J Immunol, 192(1): 523-532. 10.4049/jimmunol.1301342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Ishiwata K, Koseki H, et al. , 2010. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest, 120(8): 2867-2875. 10.1172/jci42680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walens A, Dimarco AV, Lupo R, et al. , 2019. CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. eLife, 8: e43653. 10.7554/eLife.43653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanderley CW, Colón DF, Luiz JPM, et al. , 2018. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res, 78(20): 5891-5900. 10.1158/0008-5472.Can-17-3480 [DOI] [PubMed] [Google Scholar]
- Wang FR, Li B, Wei YC, et al. , 2018. Tumor-derived exosomes induce PD1+ macrophage population in human gastric cancer that promotes disease progression. Oncogenesis, 7(5): 41. 10.1038/s41389-018-0049-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb LM, Oyesola OO, Früh SP, et al. , 2019. The Notch signaling pathway promotes basophil responses during helminth-induced type 2 inflammation. J Exp Med, 216(6): 1268-1279. 10.1084/jem.20180131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williford JM, Ishihara J, Ishihara A, et al. , 2019. Recruitment of CD103+ dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci Adv, 5(12): eaay1357. 10.1126/sciadv.aay1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa S, Oh-Hora M, Hashimoto R, et al. , 2019. Pivotal role of STIM2, but not STIM1, in IL-4 production by IL-3-stimulated murine basophils. Sci Signal, 12(576): eaav2060. 10.1126/scisignal.aav2060 [DOI] [PubMed] [Google Scholar]
- Yoshimoto T, Yasuda K, Tanaka H, et al. , 2009. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol, 10(7): 706-712. 10.1038/ni.1737 [DOI] [PubMed] [Google Scholar]
- Zellweger F, Buschor P, Hobi G, et al. , 2018. IL-3 but not monomeric IgE regulates FcεRI levels and cell survival in primary human basophils. Cell Death Dis, 9(5): 510. 10.1038/s41419-018-0526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QF, Qin JL, Zhong L, et al. , 2015. CCL5-mediated Th2 immune polarization promotes metastasis in luminal breast cancer. Cancer Res, 75(20): 4312-4321. 10.1158/0008-5472.CAN-14-3590 [DOI] [PubMed] [Google Scholar]
- Zhang SB, Zhong M, Wang C, et al. , 2018. CCL5-deficiency enhances intratumoral infiltration of CD8+ T cells in colorectal cancer. Cell Death Dis, 9(7): 766. 10.1038/s41419-018-0796-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Li BH, Yang XZ, et al. , 2008. IL-4-induced Stat6 activities affect apoptosis and gene expression in breast cancer cells. Cytokine, 42(1): 39-47. 10.1016/j.cyto.2008.01.016 [DOI] [PubMed] [Google Scholar]
- Zhang Y, He SJ, Mei RM, et al. , 2018. miR-29a suppresses IL-13-induced cell invasion by inhibiting YY1 in the AKT pathway in lung adenocarcinoma A549 cells. Oncol Rep, 39(6): 2613-2623. 10.3892/or.2018.6352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Meng WB, Yue P, et al. , 2020. M2 macrophage-derived extracellular vesicles promote gastric cancer progression via a microRNA-130b-3p/MLL3/GRHL2 signaling cascade. J Exp Clin Cancer Res, 39: 134. 10.1186/s13046-020-01626-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhao N, Dang H, Ma LC, et al. , 2021. Intratumoral γδ T-cell infiltrates, chemokine (C-C motif) ligand 4/chemokine (C-C motif) ligand 5 protein expression and survival in patients with hepatocellular carcinoma. Hepatology, 73(3): 1045-1060. 10.1002/hep.31412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZY, Jia SF, Shao CS, et al. , 2020. Irradiation induces cancer lung metastasis through activation of the cGAS-STING-CCL5 pathway in mesenchymal stromal cells. Cell Death Dis, 11(5): 326. 10.1038/s41419-020-2546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SF, Artis D, 2010. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol, 11(4): 289-293. 10.1038/ni.1852 [DOI] [PMC free article] [PubMed] [Google Scholar]