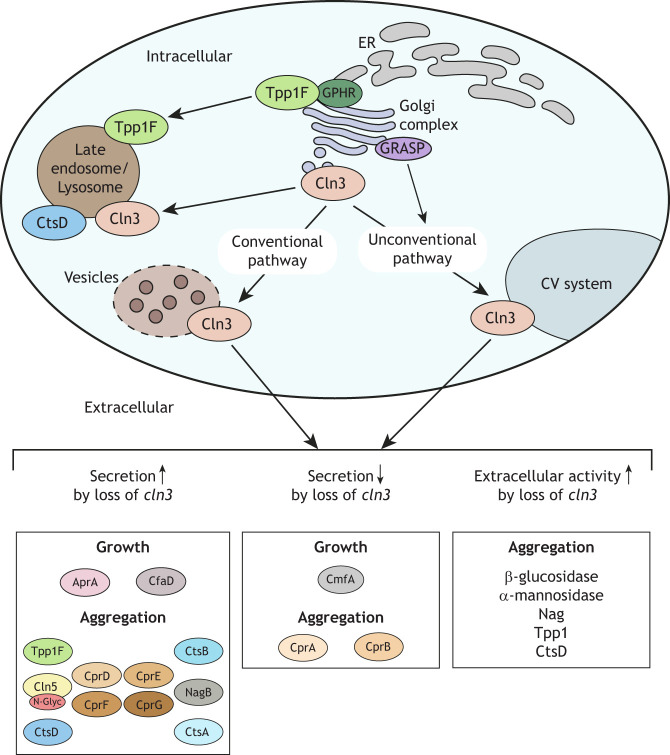
Fig. 3.
Protein secretion regulated by Cln3 in D. discoideum. Cln3 localizes to the late endosome/lysosome, together with Tpp1F and CtsD. Tpp1F also localizes to the ER and Golgi complex, and binds GPHR. For secretion via the conventional pathway, proteins are transported via the ER and Golgi complex, where they are packaged into vesicles prior to secretion. The alternative unconventional pathway involves GRASP and the CV system. Cln3 localizes to both the Golgi complex and CV system to regulate protein secretion via these pathways. Loss of cln3 increases the secretion of AprA and CfaD during growth, and Tpp1F, Cln5, CtsD, CprD, CprE, CprF, CprG, CtsB, NagB, and CadA during aggregation. Loss of cln3 decreases the secretion of CmfA during growth, and CprA and CprB during aggregation. Loss of cln3 increases the extracellular activity of beta-glucosidase, alpha-mannosidase, Nag, Tpp1, and CtsD during aggregation. Cpr, cysteine proteinase; GPHR, Golgi pH regulator (officially known as Gpr89); GRASP, Golgi reassembly-stacking protein (officially known as GrpA); N-Glyc, N-glycosylation; Tpp1F, tripeptidyl peptidase 1F.