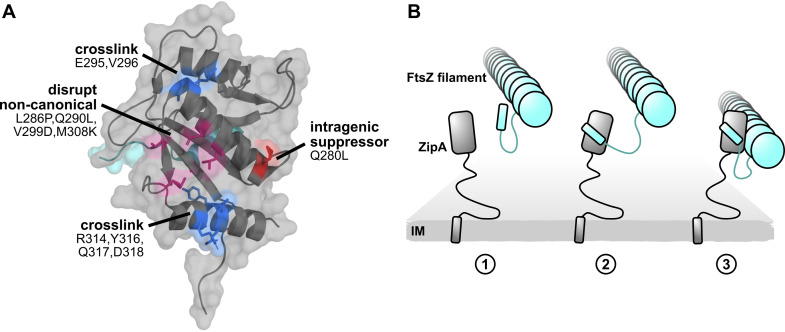
FIG 10.
A model of ZipA-FtsZ binding and function. (A) ZipA crystal structure annotated with noncanonical cross-linking residues (blue), mutations that disrupt noncanonical cross-linking (magenta), and the intragenic suppressor Q280L (red). (B) A model illustrating how two-pronged binding might occur between ZipA and FtsZ. Here the FtsZ CTP first engages with the ZipA canonical binding site. This brings ZipA and FtsZ closer so that the ZipA noncanonical interface can fully engage with the FtsZ core domain. While only one ZipA was illustrated for simplicity, multiple proteins likely engage along the length of the FtsZ filament.