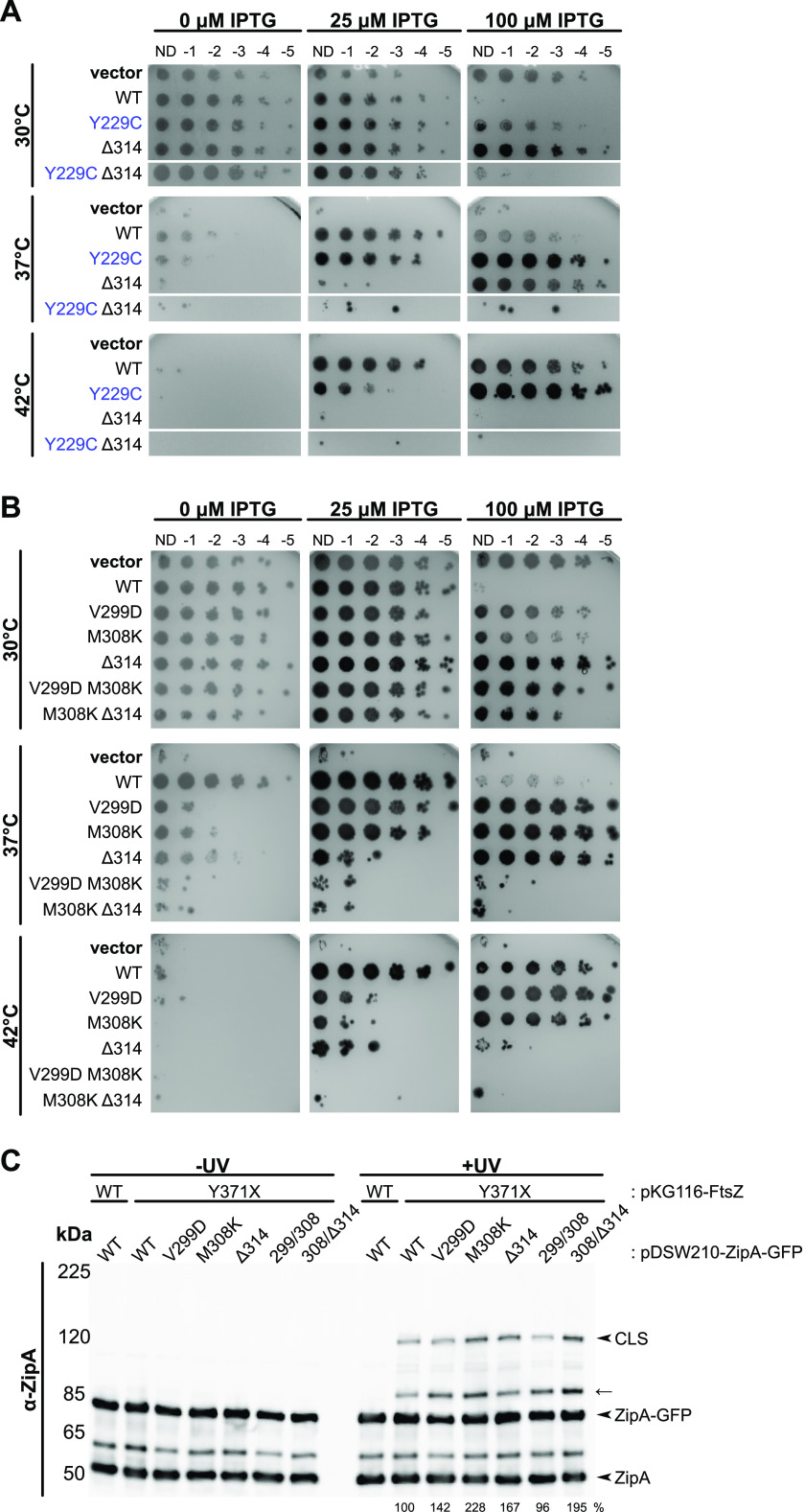
FIG 8.
Combining single residue changes in noncanonical binding residues of ZipA decreases ZipA function. Cultures of strain WM5337 (zipA1) transformed with pDSW210-GFP (vector), pDSW210-ZipA-GFP, or derived plasmids carrying zipA mutations were serially diluted and spotted on plates containing 0, 25, or 100 μM IPTG and then grown at permissive (30°C) or restrictive (37°C and 42°C) temperatures. (A) ZipA-GFP carrying both canonical (Y229C) and noncanonical (ΔR314) residue changes failed to complement zipA1 at restrictive temperatures. Panels shows composite images of two plates. (B) Combining mild noncanonical face mutations V299D and M308K together or with ΔR314 severely reduced ZipA-GFP function. (C) Bpa cross-linking in strain WM1074 carrying pUltra-pBpF, pKG116-FtsZ, and pDSW210-ZipA-GFP or derived constructs containing indicated mutations was assessed by Western blotting as described, except that cultures were induced with 25 μM IPTG. The FtsZ(Y371X) CTP maintained strong cross-linking with both single and double noncanonical site mutations. The arrow indicates probable CLS formed between FtsZ(Y371X) and native ZipA. Cross-linking efficiency relative to FtsZ(Y371X) is indicated below +UV lanes and was normalized by total protein levels and ZipA-GFP expression for each strain.