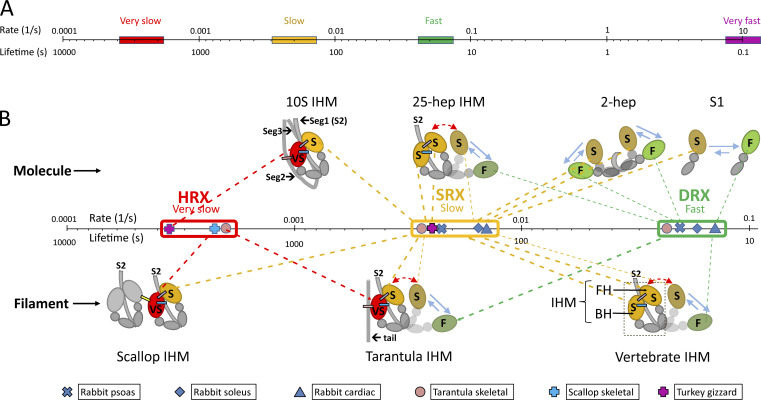
Figure 1.
ATP turnover rates of myosin and proposed relation to head organization. (A) Logarithmic plot of rates, varying from very slow (hyper-relaxed), to slow (super-relaxed), to fast (disordered-relaxed), to very fast (actin-activated). (B) Expanded plot of relaxed rates and proposed relation to head configuration and interactions. The cartoons show the IHM as found in single molecules (upper) and thick filaments (lower); in filaments, only molecules in a single crown are shown. The colored ellipses are the interacting motor domains. The smaller, gray domains are the ELCs and RLCs, and the gray lines are the proximal region of the myosin tail (S2). Molecules with a sufficient length of S2 (25 heptads [25 hep]; ∼250 Å) form an IHM, while single heads and two-headed molecules with only two heptads of tail show no interactions. Molecules in filaments have SRX and DRX turnover rates similar to those of isolated molecules (25-heptad IHM; cf. vertebrate IHM) or more inhibited (HRX) rates. Heads turn over ATP at fast (F; noninteracting), slow (S; interacting), or very slow (VS; more interactions) rates. Slow (attached) FHs of the IHM can detach and sway out (red, double-headed arrows) and, while detached, can equilibrate between the slow and fast conformations, similar to S1 (blue, reversible arrows). The “tail” in tarantula and the uncolored IHM in scallop provide additional, intermolecular interactions leading to the very slow rate; this rate is also seen in 10S myosin through intramolecular interactions with segment 2 of its own tail. The different lengths of the bars in A correspond to the lengths in B, which are drawn to include the range of rates for HRX, SRX, and DRX; the key point is the close clustering of rates within these groups and the large gaps between them.