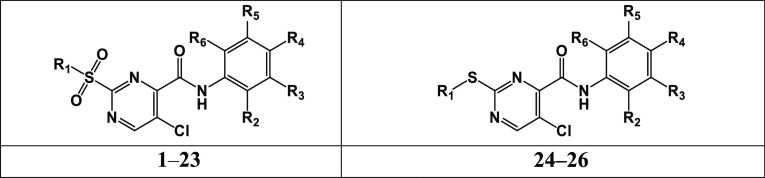
Table 4. Structure–Activity Relationships of the 2-Sulfonylpyrimidine/2-Thiopyrimidine Derivatives.
| Chembridge ID# | Cpd | R1 | R2 | R3 | R4 | R5 | R6 | IC50 (μM) [95% CI] |
|---|---|---|---|---|---|---|---|---|
| 9007737 | C9 | Me | H | I | H | H | H | 118 [92–162] |
| 9002388 | C9.1 | Me | H | H | H | H | H | 300 [204–543] |
| 7992875 | C9.2 | Me | Me | H | H | H | H | 81 [58–1009] |
| 7993471 | C9.3 | Me | Cl | H | H | H | H | 53 [45–63] |
| 7997746 | C9.4 | Me | H | Me | H | H | H | 78 [64–99] |
| 9000796 | C9.5 | Me | H | Cl | H | H | H | 45 [37–55] |
| 9331527 | C9.6 | Me | H | CF3 | H | H | H | 67 [52–91] |
| 9002023 | C9.7 | Me | H | H | OCHF2 | H | H | 34 [28–42] |
| 7999377 | C9.8 | Me | H | H | OMe | H | H | 54 [45–66] |
| 7998346 | C9.9 | Me | Me | H | H | Me | H | 102 [89–119] |
| 7994681 | C9.10 | Me | H | Me | Me | H | H | 70 [67–99] |
| 9001867 | C9.11 | Me | Me | H | H | H | Me | 186 [149–164] |
| 7992530 | C9.12 | Me | Cl | H | Cl | H | H | 19 [14–24] |
| 9002556 | C9.13 | Me | 2-naphthyl | 37 [22–34] | ||||
| 7995713 | C9.14 | Et | H | H | H | H | H | 133 [1122–147] |
| 9001771 | C9.15 | Et | F | H | H | H | H | 65 [54−83] |
| 9008307 | C9.16 | Et | H | Me | H | H | H | 79 [67−96] |
| 9005591 | C9.17 | Et | H | Cl | H | H | H | 35 [28−43] |
| 9004507 | C9.18 | Et | H | H | Me | H | H | 36 [28−46] |
| 7997031 | C9.19 | Et | H | H | Et | H | H | 24 [21−29] |
| 7997789 | C9.20 | Et | H | H | I | H | H | 23 [12−41] |
| 7994081 | C9.21 | Pr | H | Me | H | H | H | 59 [50−71] |
| 9005204 | C9.22 | Pr | H | Cl | H | H | H | 20 [17−24] |
| 7987235 | C9.23 | Me | H | I | H | H | H | 40 [25−47] |
| 9033235 | C9.24 | Me | H | Me | H | Me | H | 36 [21−56] |
| 9007737 | C9.25 | Et | H | NO2 | H | H | H | 29 [26−33] |