Abstract
Objective:
COVID-19 and associated morbidity and mortality has disproportionately affected minoritized populations. The epidemiology of spread of COVID-19 among pregnant women by race/ethnicity is not well described. Using data from a large healthcare system in California, we estimated prevalence and spread during pregnancy and recommend a vaccination approach based on minimizing adverse outcomes.
Methods:
Patients delivering at Sutter Health are tested (molecular) for COVID-19. These results were combined with antibody test results, using samples drawn at delivery. For each racial/ethnic group, we estimated prevalence of COVID-19, using logistic regression to adjust for known sociodemographic and comorbid risk factors. Testing for immunoglobulin G and immunoglobulin M provided insight into timing of infections.
Results:
Among 17,446 women delivering May–December, 460 (2.6%) tested positive (molecular). Hispanic women were at 2.6 times the odds of being actively infected as White women (odds ratio = 2.6, 95% confidence interval = 2.0–3.3). August and December were the highest risk periods for active infection (odds ratio = 3.5, 95% confidence interval = 2.1–5.7 and odds ratio = 6.1, 95% confidence interval = 3.8–9.9, compared with May, respectively). Among 4500 women delivering October–December, 425 (9.4%) had positive molecular or antibody tests, ranging from 4.0% (Asian) to 15.7% (Hispanic). Adjusting for covariables, compared with White patients, odds of infection was similar for Black and Asian patients, with Hispanic at 2.4 (1.8–3.3) times the odds.
Conclusion:
COVID-19 prevalence was higher among Hispanic women at delivery and in the last trimester than their White counterparts. Higher rates in Black patients are explained by other risk factors. Resources should be directed to increase vaccination rates among Hispanic women in early stages of pregnancy.
Keywords: antibodies, COVID-19, COVID-19 disparities, COVID-19 vaccine strategy, health equity, pregnancy
Introduction
Across the US rates of adverse maternal childbirth events differ substantially by race and ethnicity, with mothers from minoritized communities at higher risk of morbidity and mortality.1–4 Similarly, the COVID-19 pandemic has disproportionately affected some minoritized populations both in terms of severity and outcomes.5–13
Recent studies have suggested that because pregnant women are at greater risk of severe illness from other respiratory infections, such as the flu, it is likely that similar risk exists for COVID-19. 14 Furthermore, once infected, there is evidence of increased maternal morbidity and adverse neonatal outcomes.11,15–18
Despite increased availability of testing, data on rates of COVID-19 in the pregnant population remain limited; however, many hospitals and healthcare systems have implemented standardized testing protocols for their parturient patients. On 1 May 2020, Sutter Health implemented a systemwide policy to test every labor and delivery patient, upon admission, using molecular testing. 19 Such data provide insight about COVID-19 prevalence at admission, regardless of symptoms. However, the time period from conception to delivery still represents a knowledge gap and could provide critically important epidemiologic data about the disease.
The emergence of the more virulent delta variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), currently the dominant strain of the virus that causes COVID-19 in the United States, 20 underscores the need for us to both understand which groups of pregnant women are most affected by the spread of COVID-19 and to develop strategies to intervene. 21 With this knowledge, we can develop and implement a vaccination strategy specifically designed to increase vaccination rates for these high-risk patients.
To address this knowledge gap, we tested parturient women, delivering at Sutter Health hospitals from October to December 2020, for antibodies to SARS-CoV-2. We combined antibody data with the results of molecular tests to form a more complete picture of the patterns of the disease among pregnant women and address the following objectives:
- Objective 1: Among those patients who delivered from 1 October 2020 to 31 December 2020
- (a) To estimate overall infection with COVID-19 during pregnancy and within this time period as assessed by either positive molecular test or positive antibody test at date of delivery.
- (b) To test for differential COVID-19 infection rates in patients giving birth by race and ethnicity, adjusting for age and other risk factors, including comorbidities associated with pregnancy, method of delivery, parity, and insurance type.
Objective 2: To estimate the rates of positive COVID-19 molecular tests over time among women giving birth between 1 May 2020 and 31 December 2020.
Objective 3: To calculate the Covid-19 Vaccine Equity Index (CVEI) 22 for pregnant women to identify subgroups to target for vaccine outreach.
Methods
Setting
This observational study was conducted at Sutter Health, a large mixed-payer, integrated healthcare delivery system in northern California. Sutter delivers comprehensive medical services in more than 100+ ambulatory clinics and 23 acute care hospitals, caring for approximately 3.5 million people each year, across 22 counties in both urban and rural settings. 23 Ten of those counties are in the San Francisco Bay Area, a highly populated and racially diverse region. In 2020, 27,493 babies were born at Sutter birthing centers across the system. 24 Sutter’s electronic health record (EHR; Epic Systems Corporation) is fully integrated across all hospital and ambulatory sites. Sutter has collected patient self-reported race, ethnicity, ancestry, and language (REAL) since 2010. As of 2020, Sutter Health patients were by self-report, 45.6% non-Hispanic White (NHW), 15.6% Hispanic, 16.5% non-Hispanic Asian (NHA), 4.7% non-Hispanic Black (NHB), and 17.4% other (American Indian/Alaskan Native, mixed race, declined-to-state, and unknown). We use the designation “Hispanic,” instead of the commonly used term “LatinX,” for consistency with US Census categories and Sutter Health self-reported data collection. This study was approved by the healthcare system’s Institutional Review Board (IRBNet # 1651594-4) and was conducted according to Health Insurance Portability and Accountability Act standards.
Blood specimens
Blood specimens collected for clinical purposes into ethylenediamine tetraacetic acid (EDTA) tubes from women who gave birth at one of 14 participating Sutter hospital birthing centers were stored at 2–8°C for 6 days. Blood samples were then centrifuged and plasma was aliquoted into clean test tubes and frozen at −20°C until testing. The 14 birthing centers agreed to participate in this research project and accounted for 90.6% of all births during the time period. All patients who presented for delivery and had stored blood samples were included in the study; those with missing or quantity insufficient for aliquoting were excluded from the analysis. Using an anonymization crosswalk shared between the data steward and the Transfusion Services, these samples were relabeled with a STUDYID and sent to the Clinical lab to be tested for SARS-CoV-2 antibodies. Anonymized results (identified only by STUDYID) were returned directly to the study investigators. In parallel, the data steward extracted patient demographic and clinical EHR data to create a limited data set (LDS), anonymized and identified only by STUDYID. This anonymized LDS was also shared with investigators.
COVID-19 molecular testing
Following a system-wide Sutter Health policy, all labor and delivery patients were tested by a molecular method for COVID-19 upon admission or up to 3 days before admission. Nasopharyngeal or nasal mid-turbinate swabs were collected and tested on-site at the acute care facility or were sent to Sutter Health’s Shared Laboratory. Molecular testing for the detection of SARS-CoV-2 RNA was performed on one of the following testing platforms granted emergency use authorization (EUA) by US Food and Drug Administration (FDA), following the manufacturer’s instructions: Roche Cobas 6800, Hologic Panther Aptima, Hologic Panther Fusion, Luminex Aries, DiaSorin LIAISON MDX, Cepheid GeneXpert, Roche Liat, or Abbott ID Now.
Antibody testing
Residual EDTA plasma samples were tested for SARS-CoV-2 total antibodies using the Siemens Atellica platform. The Atellica assay is a chemiluminescent immunoassay that detects total antibodies (including immunoglobulin G (IgG) and immunoglobulin M (IgM)) against the receptor-binding domain (RBD) of the viral spike protein. Samples positive for total antibodies were stored at −20°C for a maximum period of 3 months until further specific serology testing was performed. Both IgG-specific and IgM-specific chemiluminescent immunoassays were performed on the DiaSorin LIAISON XL analyzer. The IgG assay is designed to detect IgG antibodies against the full-length spike protein (S1/S2), while the IgM assay detects IgM antibodies against RBD. See Online Appendix 1 for testing accuracy metrics.
Cohorts and EHR data set
We used a different cohort to address each objective, leveraging the maximum available data for each. The three research databases were constructed as follows:
EHR data, molecular test results, and antibody test results for the study period of 1 October 2020–31 December 2020, plus a 9-month EHR-based look back window for each of 4500 patients in the COVID-19 Antibody Pregnancy Epidemiology study.
EHR data (demographics, comorbidity, birth factors) and molecular test lab data from 1 May 2020 to 31 December 2020 for 17,446 parturient patients delivering at Sutter Health facilities during the study period.
EHR data from Sutter for March 2020–March 2021 for women of childbearing age (16–45 years) who had a positive molecular test for COVID-19.
Covariates
We extracted demographic information from the EHR for all patients, including patients’ dates of birth, sex, self-reported race and ethnicity, and primary insurance. Age was classified into three groups: ⩽25, 26–34, and ⩾35 years. Race and ethnicity were defined by Hispanic identity, followed by racial group. If a patient did not self-identify as Hispanic/LatinX, we classified them based on their race. Insurance status was identified by the active primary payer documented in the EHR, at the most recent encounter, and classified as commercial, public (Medicaid, federal, state, and local government non-Medicare insurance plans, Medicare Part A/B or Part C), or other/self-pay/not reported.
From the EHR, we extracted patients’ comorbidities, using International Classification of Diseases, 10th Revision (ICD-10) diagnoses as of the date of delivery, including hypertension, gestational diabetes mellitus (GDM), and preeclampsia. We also extracted mother’s blood type, the mode of delivery, and number of previous live births (parity). Parity was further classified as yes (at least one previous live birth) versus no (nulliparous). Mode of delivery was classified as cesarean (C-section) versus vaginal birth (Y/N).
COVID-19 molecular test results and hospital admission data extracted for all women of childbearing age (cohort 3) were used in CVEI calculations related to hospitalization rates.
Analysis and statistical methods
The primary analysis was an estimation of the prevalence of antibodies (IgG for all, and both IgG and IgM for some) by age and race/ethnicity. Antibodies, including IgM and IgG as well as immunoglobulin A (IgA), can be detected within 1–3 weeks after COVID infection. COVID IgM and IgG usually arise within a short period of one another, and in some cases, IgG and IgM can arise almost simultaneously. IgM does degrade more rapidly (within about 6 weeks–2 months) than IgG which generally persists for months. Therefore, we considered the presence of IgM antibodies as an indicator of a more recent infection—6 weeks–2 months prior.25–27
We used logistic regression to estimate the odds of infection for each racial/ethnic subgroup and used multivariable logistic regression to adjust for demographics, month of delivery, and maternal comorbidities.
To estimate the rates of positive COVID-19 molecular tests over time among women giving birth between 1 May 2020 and 31 December 2020, we used molecular testing results to measure active virus at the time of delivery for pregnant women over part of the course of the pandemic in the Sutter Health population to determine how the burden of virus varied over time and by race and ethnicity. We graphed proportions over time and used multivariable logistic regression to adjust for covariates.
No statistical power calculation was conducted prior to the start of the study because we analyzed all available data.
COVID-19 Vaccine Equity Index
Because there are now extremely safe and efficacious vaccines available for use during pregnancy,21,28 vaccination has become the most effective tool to arrest spread of the disease.
We previously developed the CVEI 22 as a practical tool intended to provide insight into the impact of vaccination strategies on addressing disparities, promoting more equitable outcomes among racial/ethnic groups, and reducing overall morbidity and mortality within the population as we pursue the collective goal of herd immunity through mass vaccination. The index is designed to enhance equity, the attainment of equivalent outcomes among all subgroups. This is more complex than equality which simply reflects the degree to which the same actions are applied to every subgroup. If we vaccinate all racial/ethnic subgroups equally, the likelihood of being vaccinated (or conversely the likelihood of being unvaccinated) is the same for all subgroups. However, equality does not address the impact of the disproportionate burden of adverse outcomes borne by certain groups.
The CVEI indicates whether equity (as measured by observing the same outcomes) has been achieved for a given subgroup and its value reflects the magnitude of the disparity. It is calculated in steps by taking the product of three risk ratios
The ratios inform the specific actions we may be able to take to reduce that disparity. Elevations in R1 reflect equality of our vaccination efforts, whereas R2 and R3 help identify which members of the subgroup, if vaccinated, would have the greatest impact on reducing the disparity. For example, elevations in R2 suggest that vaccinating members of the subgroup most at risk of becoming infected would improve measures of equity. Elevations in R3 suggest that preferentially vaccinating subgroup members at greatest risk for severe illness and hospitalization, if infected, is a strategy that will have the maximum impact on improving equity. We calculated the CVEIs for each racial/ethnic subgroup(s), assuming a vaccine equality target of 70% vaccinated to achieve a goal of herd immunity. 29
To calculate R1, we used the proportion of primary care female patients of childbearing age who were unvaccinated as of September 2021 by race and ethnicity as a proxy which reflects vaccination rates in pregnant women. R1 is the ratio of these subgroup proportions to the proportion unvaccinated overall. To calculate R2, we used Cohort 1 and constructed a multivariable logistic regression predicting the odds of infection measured by either molecular or antibody test among parturient women. This model was adjusted for age, insurance type (public vs private), and relevant comorbidities associated with pregnancy and/or COVID-19 (diabetes, hypertension, preeclampsia, C-section, parity, and blood type). From this model, we calculated adjusted probabilities of a positive test during pregnancy and compared them with the overall adjusted probability. Calculation of accurate COVID-related hospitalization rates for this cohort was challenging because pregnant women are frequently hospitalized for a range of non-COVID-related issues. Therefore, as a proxy, we used data from cohort 3 (women of childbearing age with a positive COVID-19 molecular test) to estimate hospitalization rates and R3. We adjusted these models for age, insurance type (public vs private), and relevant comorbidities associated with severe COVID-19 (diabetes, hypertension, congestive heart failure, cardiovascular disease, chronic obstructive pulmonary disease, asthma, alcohol disorder, and obesity).
Results
Cohort 1: patients who gave birth from 1 October 2020 to 31 December 2020
From 1 October to 31 December, 4500 patients delivered babies and had acceptable discarded plasma to test. Of those, 159 (3.5%) had positive molecular tests at delivery, 363 (8.1%) had positive antibody results, and overall 425 (9.4%) had evidence of current or prior infection (Table 1). Proportions of those with any evidence of infection differed by race, ranging from 4% (n = 28) for NHA to 15.7% (n = 239) for Hispanic patients. Among NHB patients, 10.8% (n = 35) had evidence of infection, and among NHW, 5.0% (n = 77).
Table 1.
Descriptive demographics and clinical variables by COVID-19 status—cohort 1 October 2020 to December 2020.
| Factor | Total | No COVID | COVID-19 infection | p-value |
|---|---|---|---|---|
| N = 4500 | n = 4075 | n = 425 | ||
| Age (years) | 30.9 ± 5.7 | 31.1 ± 5.7 | 28.9 ± 6.1 | <0.001 |
| Age | <0.001 | |||
| 1: ⩽25 | 859 (19.1%) | 722 (17.7%) | 137 (32.2%) | |
| 2: 26–34 | 2632 (58.5%) | 2414 (59.2%) | 218 (51.3%) | |
| 3: 35+ | 1009 (22.4%) | 939 (23.0%) | 70 (16.5%) | |
| Race/ethnicity | <0.001 | |||
| NH White | 1539 (34.2%) | 1462 (35.9%) | 77 (18.1%) | |
| Hispanic | 1525 (33.9%) | 1286 (31.6%) | 239 (56.2%) | |
| NH Black | 325 (7.2%) | 290 (7.1%) | 35 (8.2%) | |
| NH Asian | 705 (15.7%) | 677 (16.6%) | 28 (6.6%) | |
| Other | 406 (9.0%) | 360 (8.8%) | 46 (10.8%) | |
| Insurance | <0.001 | |||
| Commercial | 2239 (49.8%) | 2137 (52.4%) | 102 (24.0%) | |
| Public | 1970 (43.8%) | 1666 (40.9%) | 304 (71.5%) | |
| Other/self/unknown | 291 (6.5%) | 272 (6.7%) | 19 (4.5%) | |
| Month | <0.001 | |||
| October | 1586 (35.2%) | 1481 (36.3%) | 105 (24.7%) | |
| November | 1492 (33.2%) | 1345 (33.0%) | 147 (34.6%) | |
| December | 1422 (31.6%) | 1249 (30.7%) | 173 (40.7%) | |
| Hypertension | 0.17 | |||
| No | 4464 (99.2%) | 4040 (99.1%) | 424 (99.8%) | |
| Yes | 36 (0.8%) | 35 (0.9%) | 1 (0.2%) | |
| GDM | 0.40 | |||
| No | 4056 (90.1%) | 3668 (90.0%) | 388 (91.3%) | |
| Yes | 444 (9.9%) | 407 (10.0%) | 37 (8.7%) | |
| Preeclampsia | 0.80 | |||
| No | 4299 (95.5%) | 3894 (95.6%) | 405 (95.3%) | |
| Yes | 201 (4.5%) | 181 (4.4%) | 20 (4.7%) | |
| Birth type | 0.24 | |||
| Vaginal | 3388 (75.3%) | 3058 (75.0%) | 330 (77.6%) | |
| C-section | 1112 (24.7%) | 1017 (25.0%) | 95 (22.4%) | |
| Parity | <0.001 | |||
| Nulliparous | 1735 (38.6%) | 1616 (39.7%) | 119 (28.0%) | |
| 1+ previous | 2765 (61.4%) | 2459 (60.3%) | 306 (72.0%) | |
| Blood type | 0.048 | |||
| O | 2204 (49.0%) | 1984 (48.7%) | 220 (51.8%) | |
| A | 1443 (32.1%) | 1297 (31.8%) | 146 (34.4%) | |
| AB | 198 (4.4%) | 185 (4.5%) | 13 (3.1%) | |
| B | 655 (14.6%) | 609 (14.9%) | 46 (10.8%) |
NH: non-Hispanic; GDM: gestational diabetes mellitus.
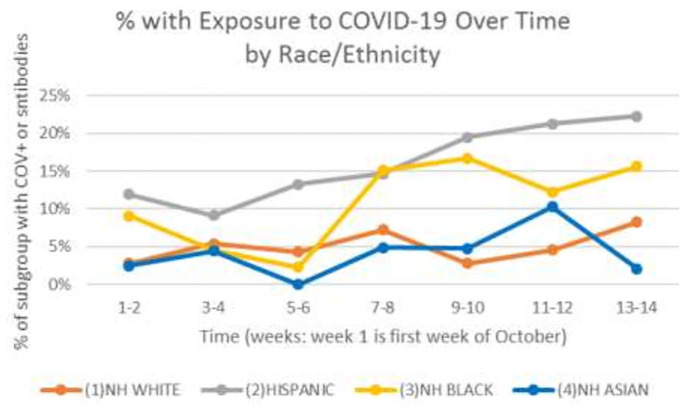
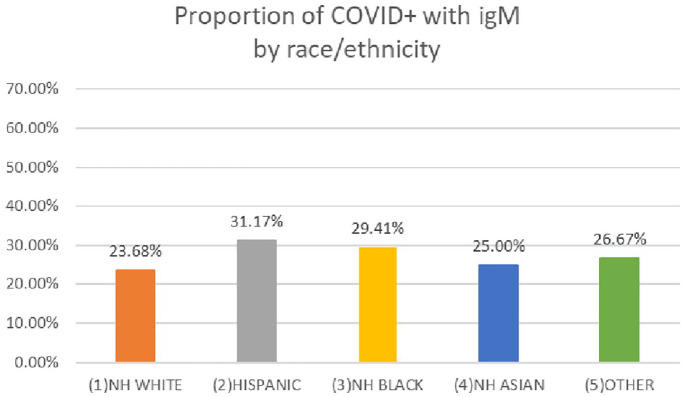
In the unadjusted logistic regression, Hispanic patients were at increased odds of having current or prior infection (odds ratio (OR) = 3.5; 95% confidence interval (95% CI) = 2.7–4.6), as were NHB patients (OR = 2.3; 95% CI = 1.5–3.5); however, NHA patients had similar odds compared with NHW patients (OR = 0.8; 95% CI = 0.5–1.2) (Table 2). In models adjusted for age and insurance type, Hispanic patients remained at significantly increased odds (OR = 2.4; 95% CI = 1.8–3.2), but NHB patients were no longer significantly different from their NHW counterparts. In fully adjusted models, Hispanic patients had 2.4 times greater odds of being currently or previously infected (95% CI = 1.8–3.2) and other factors associated with increased odds were young age, month of delivery (for November: OR = 1.7; 95% CI = 1.3–2.2; for December: OR = 2.1; 95% CI = 1.6–2.7), having public insurance (OR = 2.5; 95% CI = 1.9–3.2), and parity (OR = 1.6; 95% CI = 1.3–2.1). Figure 1 shows percentages of delivering mothers who had either current or previous infection by race and ethnicity over time. From this figure, for Hispanic mothers, the likelihood of infection increased over time, while it was stable for NHA and NHW mothers. For NHB mothers, there was a sharp increase in November, followed by a plateau through the end of the year. Among those in cohort 1 who tested positive for SARS-CoV-2 antibodies, the proportion with IgM antibodies ranged from 23.7% for NHB patients to 31.2% for Hispanic patients. See Figure 2. The presence of IgM antibodies suggests that exposure to the virus occurred within the third trimester.
Table 2.
Odds ratios for COVID-19 infection (either current or previous) and 95% confidence intervals—cohort 1 October 2020–December 2020.
| Factor | Univariate | +Age | +Insurance | Full model |
|---|---|---|---|---|
| N = 4500—with 425 COVID-19 cases | ||||
| Race/ethnicity | ||||
| NH White | 1.00—ref | 1.00—ref | 1.00—ref | 1.00—ref |
| Hispanic | 3.53 (2.70–4.61) | 3.20 (2.44–4.20) | 2.40 (1.81–3.17) | 2.37 (1.78–3.16) |
| NH Black | 2.29 (1.51–3.48) | 2.09 (1.37–3.20) | 1.46 (0.94–2.24) | 1.47 (0.95–2.27) |
| NH Asian | 0.79 (0.50–1.22) | 0.80 (0.52–1.25) | 0.73 (0.47–1.14) | 0.78 (0.49–1.22) |
| Other | 2.43 (1.65–3.56) | 2.25 (1.53–3.31) | 1.78 (1.20–2.64) | 1.79 (1.20–2.66) |
| Age (years) | ||||
| ⩽25 | 1.00—ref | 1.00—ref | 1.00—ref | |
| 26–34 | 0.60 (0.47–0.76) | 0.72 (0.57–0.92) | 0.62 (0.48–0.81) | |
| 35+ | 0.55 (0.40–0.75) | 0.71 (0.51–0.98) | 0.57 (0.40–0.81) | |
| Insurance | ||||
| Private/commercial | 1.00—ref | 1.00—ref | ||
| Public | 2.72 (2.12–3.50) | 2.48 (1.91–3.21) | ||
| Other/self/unknown | 1.16 (0.70–1.94) | 1.06 (0.63–1.78) | ||
| Date | ||||
| October | 1.00—ref | |||
| November | 1.70 (1.30–2.22) | |||
| December | 2.08 (1.60–2.71) | |||
| Comorbidities | ||||
| Hypertension | 0.26 (0.04–1.98) | |||
| GDM | 0.86 (0.59–1.25) | |||
| Preeclampsia | 1.03 (0.63–1.69) | |||
| C-section | 0.97 (0.75–1.24) | |||
| Parity a | 1.61 (1.25–2.06) | |||
| Blood type | ||||
| O | 1.00—ref | |||
| A | 1.12 (0.89–1.41) | |||
| AB | 0.84 (0.46–1.54) | |||
| B | 0.85 (0.60–1.20) | |||
NH: non-Hispanic; GDM: gestational diabetes mellitus.
Reference group is nulliparous.
Figure 1.
Any exposure to COVID-19 (current or previous) by race and ethnicity over time in weeks from the first week in October 2020 to the last week in December 2020. Data are from cohort 1.
Figure 2.
Proportion of patients with COVID-19 who tested positive for IgM antibodies by race and ethnicity. Data are from cohort 1, October 2020–December 2020.
Cohort 2: patients who gave birth from 1 May 2020 to 31 December 2020
From 1 May to 31 December, 17,446 patients delivered babies across the 18 birthing centers at Sutter Health. Of those, 460 (2.6%) had positive molecular tests at delivery. Proportions differed by race as follows: 1.3% (n = 78) for NHW, 4.7% (n = 286) for Hispanic, 2.2% (n = 25) for NHB, and 1.1% (n = 29) for NHA. In the unadjusted logistic regression, Hispanic patients were at nearly 4 times the odds of testing positive at delivery (OR = 3.80; 95% CI = 2.95–4.89) compared with NHW patients (see Table 3). NHB patients were also at increased odds compared with NHW (OR = 1.8; 95% CI = 1.1–2.8). However, NHA patients did not differ significantly from NHW. After adjusting for age, insurance type, date of delivery, comorbidities, type of birth, parity, and blood type, for Hispanic patients, the odds of having an active COVID-19 infection were still elevated (OR = 2.6; 95% CI = 2.0–3.3).
Table 3.
Odds ratios for COVID-19 infection active at delivery and 95% confidence intervals for progressive models, cohort 2 May 2020–December 2020.
| Factor | Univariate | +Age | +Insurance | Full model |
|---|---|---|---|---|
| N = 17,446—with 460 COVID-19 cases | ||||
| Race/ethnicity | ||||
| NH White | 1.00—ref | 1.00—ref | 1.00—ref | 1.00—ref |
| Hispanic | 3.80 (2.95–4.89) | 3.46 (2.68–4.47) | 2.65 (2.03–3.45) | 2.55 (1.95–3.33) |
| NH Black | 1.76 (1.12–2.77) | 1.58 (1.00–2.50) | 1.12 (0.71–1.79) | 1.09 (0.68–1.74) |
| NH Asian | 0.82 (0.53–1.26) | 0.85 (0.55–1.30) | 0.78 (0.51–1.21) | 0.85 (0.55–1.31) |
| Other | 2.30 (1.57–3.36) | 2.14 (1.46–3.13) | 1.69 (1.15–2.48) | 1.69 (1.14–2.49) |
| Age | ||||
| ⩽25 | 1.00—ref | 1.00—ref | 1.00—ref | |
| 26–34 | 0.65 (0.53–0.80) | 0.78 (0.63–0.97) | 0.68 (0.54–0.85) | |
| 35+ | 0.52 (0.39–0.70) | 0.69 (0.51–0.93) | 0.57 (0.42–0.79) | |
| Insurance | ||||
| Private/commercial | 1.00—ref | 1.00—ref | ||
| Public | 2.46 (1.95–3.09) | 2.18 (1.73–2.75) | ||
| Other/self/unknown | 1.42 (0.89–2.26) | 1.30 (0.81–2.07) | ||
| Date | ||||
| May | 1.00—ref | |||
| June | 1.02 (0.55–1.89) | |||
| July | 2.23 (1.32–3.78) | |||
| August | 3.48 (2.11–5.71) | |||
| September | 3.00 (1.81–4.98) | |||
| October | 1.94 (1.13–3.32) | |||
| November | 3.30 (1.99–5.48) | |||
| December | 6.11 (3.77–9.89) | |||
| Comorbidities | ||||
| Hypertension | 1.18 (0.47–2.97) | |||
| GDM | 0.87 (0.63–1.22) | |||
| Preeclampsia | 1.43 (0.97–2.12) | |||
| C-section | 0.89 (0.71–1.11) | |||
| Parity | 1.57 (1.25–1.95) | |||
| Blood type | ||||
| O | 1.00—ref | |||
| A | 0.94 (0.76–1.17) | |||
| AB | 0.70 (0.39–1.27) | |||
| B | 0.84 (0.61–1.15) | |||
NH: non-Hispanic; GDM: gestational diabetes mellitus.
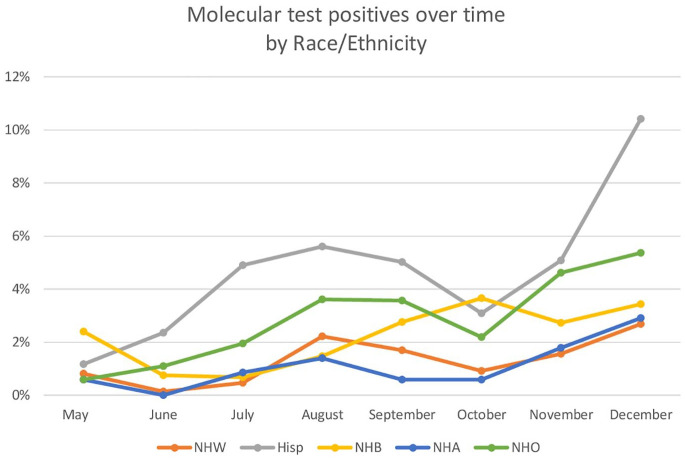
Other factors associated with increased odds were young age, month of delivery (OR for August compared with May = 3.5; 95% CI = 2.1–5.7; OR for December: 6.1; 95% CI = 3.8–9.9), having public insurance (OR = 2.2; 95% CI = 1.7–2.8), and parity (OR = 1.6; 95% CI = 1.3–2.0). Figure 3 shows percentages of delivering mothers who tested positive for COVID-19 at delivery by race and ethnicity over time. From this figure, it is apparent that for most of the year, higher percentages of Hispanic patients tested positive for COVID-19 than other racial/ethnic groups. In addition, this population had a greater surge than other racial/ethnic groups.
Figure 3.
Current COVID-19, assessed by molecular testing, reported by race and ethnicity by month of delivery from May 2020 to December 2020. Data are from cohort 2.
Cohort 3: women aged 16–45 at Sutter Health who tested positive for COVID-19
Using available COVID-19 data from January 2020 to March 2021, we conducted multivariable logistic regressions to quantify relative odds of hospitalization for infected patients. Data, summarized in Table 4, indicate that NHB women of childbearing age were at 30% greater odds of hospitalization, if infected (OR = 1.34, 95% CI = 1.01–1.79). Hispanic women were at 50% greater odds of being hospitalized, if infected (OR = 1.50, 95% CI = 1.26–1.79).
Table 4.
Adjusted probabilities for calculating the COVID-19 Vaccine Equity Index.
| Race/ethnicity | Infection (cohort 1) | Hospitalized (cohort 3) | ||
|---|---|---|---|---|
| Adjusted model | Adjusted probabilities | Adjusted model | Adjusted probabilities | |
| OR (95% CI) | OR (95% CI) | |||
| NH White | 1.00—ref | 6.09% | 1.00—ref | 2.40% |
| Hispanic | 2.37 (1.78–3.16) | 14.94% | 1.50 (1.26–1.79) | 4.55% |
| NH Black | 1.47 (0.95–2.27) | 10.11% | 1.34 (1.01–1.79) | 4.15% |
| NH Asian | 0.78 (0.49–1.22) | 5.21% | 1.51 (1.16–1.98) | 3.18% |
| Other | 1.79 (1.20–2.66) | 11.48% | 0.98 (0.75–1.29) | 2.18% |
OR: odds ratio; CI: confidence interval; NH: non-Hispanic.
Using the CVEI calculations, Table 5 shows the target vaccine percentages for pregnant women by race and ethnicity required to achieve equity. Equity target rates for Hispanic women (84%) and NHB women (74%) are higher than for NHA (34%) and NHW (25%) women and higher than the overall herd immunity target (70%).
Table 5.
CVEI calculations.
| Race/ethnicity | Ratio 1 | Ratio 2 | Ratio 3 | CVEI | Equality target | Equity target | Vaccine target |
|---|---|---|---|---|---|---|---|
| NH White | 1.19 | 0.58 | 0.69 | 0.48 | 70% | 25% | 70% |
| Hispanic | 1.06 | 1.41 | 1.31 | 1.96 | 70% | 84% | 84% |
| NH Black | 1.28 | 0.96 | 1.20 | 1.48 | 70% | 74% | 74% |
| NH Asian | 0.64 | 0.49 | 0.92 | 0.29 | 70% | 34% | 70% |
| Other | 0.99 | 1.09 | 0.63 | 0.68 | 70% | 56% | 70% |
CVEI: COVID-19 Vaccine Equity Index; NH: non-Hispanic.
Discussion
To describe the spread of COVID-19, we identified those acutely infected, as indicated by molecular testing, as well as those previously infected, evaluated by antibody testing. As with cohort 2, among the 4500 women for whom we had valid serology analysis (cohort 1), the odds of infection were 3½ times greater for Hispanic women, and more than 2 times greater for NHB women compared with NHW women. However, the differences for NHB patients were explained in large measure by their insurance status; a larger proportion were covered by Medicaid insurance, itself correlated with higher rates of infection. In adjusted models, NHW, NHB, and NHA women had similar exposure rates, while exposure rates for Hispanic women remained high (OR = 2.37). As with acute infections, younger age (<25 years), multiparity and Medicaid insurance were associated with significantly higher rates of past or current infection. Mirroring trends in positivity rates observed in the general population, peak rates for parturient patients were observed in August and December.
We also found an increasing trend of infections among Hispanic women over the study period. Of note, for all women who had been exposed to COVID-19, 40% either had active infection or IgM antibodies at delivery, suggesting that the infection likely occurred within 6 weeks prior to delivery.
Defining the spread of COVID-19 among pregnant women by race and ethnicity provides useful insight into selective interventions to reduce the spread of infection and ensuing mortality. The delta variant of SARS-CoV-2 is now the dominant strain within the United States and replicates far more rapidly than previously encountered strains. 20 Vaccination is the most effective tool to stop transmission and must be accomplished quickly to limit further viral mutation.30,31
The CVEI gives us insight into how to use our findings about spread of infection to develop a vaccination strategy that not only achieves the goal of reaching herd immunity through mass vaccination, but also addresses disparities in outcomes. Pregnant Hispanic women are at far greater risk than their non-Hispanic counterparts of being acutely infected with COVID-19 at delivery or being exposed to the virus during pregnancy. In evaluating women of childbearing age (cohort 3), after adjusting for all available risk factors, both Hispanic and NHB women were more likely than NHW women to be hospitalized, if infected. For these subgroups, the product of the ratios R2 and R3 resulted in a CVEI greater than one, indicating a disparity in outcomes. Assuming a collective goal of vaccinating 70% of all pregnant women, the CVEI would indicate that our strategy should be to set higher rates for Hispanic (84%) and NHB (74%) women. In addition, for Hispanic women, we should focus on those at highest risk of becoming infected (elevated R2) as well as those at greatest risk of hospitalization (elevated R3). For NHB women, we should focus heavily on those at greatest risk for hospitalization (elevated R3). Recognizing that a large proportion of infections were observed during the last half of the third trimester, it would also seem prudent to focus efforts on vaccinating women as early as clinically appropriate.21,32 With the arrival of the delta variant, the herd immunity target is even higher than 70%.29,33 The recommended CVEI subgroup vaccination rates strive for vaccinating 70% of all pregnant women while also producing equity of outcomes and minimizing adverse events. With this framework, vaccine targets for some subgroups may exceed 70%, while for others, calculated rates are substantially lower. While we do not advocate suppressing vaccination rates for subgroups who experience better than average outcomes, we strongly support strategies that deploy the resources necessary to achieve these higher rates in adversely impacted groups. We believe this is a useful approach for large health systems or public agencies to adopt in developing vaccination strategies for pregnant women to contain spread and mitigate disparities.
Strengths and limitations
There are a few potential limitations to the interpretation of data and results from this study. First, because we used residual blood specimens, we had to exclude 24.3% of the women who delivered at the participating birthing centers due to limited sample. However, while this significantly reduced the sample size, we believe these data are “missing completely at random” and therefore would not bias our findings. 34 Second, although the Sutter Health database is extensive and includes data for a very large number of pregnant patients, Sutter Health is an open healthcare system. Hence, some patients may have received testing at an outside facility, with results potentially not captured in our database. However, there is a well-defined process for outside medical information to be incorporated into the EHR at the time of any subsequent encounter. Because pregnant women who deliver at Sutter Health are required to interface with the system multiple times during pregnancy and prior to delivery, it is highly likely that any outside clinical data would be brought into the database as part of the patient record at those times. If, for any reason, a test result is not incorporated, this would produce an undercounting of cases and the resulting estimates would be conservative. However, we expect that any undercounting would not be disproportionate by race or ethnicity and would not affect findings having to do with racial/ethnic differences. Third, we used data from women of childbearing age as a proxy for estimating hospitalization rates in pregnant women. Although absolute estimates of rates may differ slightly between women of childbearing age and those who are pregnant, in the context of the CVEI, it is relative rates that are determinative. Women of childbearing age are representative of the population of women who might be pregnant or become pregnant and we expect that within this large sample size, relative rates between racial and ethnic groups will mirror results for pregnant women. Finally, due to sample size constraints, we were unable to evaluate some of the smaller minoritized population groups that were combined into the “NH Other” category. However, that group made up less than 10% of our study sample, had the second highest vaccination rate, and had a CVEI <1.0, indicating that it would not be a group for which we would focus additional resources.
The study also has several strengths. First, the study was conducted in a large healthcare system in northern California, and the diversity of Sutter’s patient population reflects that of the United States. Also, the large number of parturient patients included in cohort 1 uniquely allows us to estimate prevalence of COVID-19 in this population. The large sample size of cohort 2 also contributes to the robustness of our statistical observations. Our racial/ethnic classifications are based on self-reported data, generally considered the gold standard. 35 Finally, Sutter has an integrated systemwide EHR that allows us to compare and analyze standardized data across our network of birthing centers.
Conclusion
Using molecular test results combined with antibody testing to quantify prevalence of COVID-19 infection over the course of pregnancy provides insight into disparities during pregnancy. Using these data to derive the CVEI provides the opportunity to target vaccination efforts for pregnant women and minimize adverse outcomes. With ever-increasing rates of infection with the delta variant, the need to vaccinate strategically to reduce infections, hospitalizations, and deaths among the unvaccinated is critical. This approach also protects those who are vaccinated by preventing the emergence of other even more dangerous variants.
Supplemental Material
Supplemental material, sj-docx-1-whe-10.1177_17455065211063300 for COVID-19 in pregnancy by race and ethnicity: Implications for development of a vaccination strategy by Alice Pressman, Stephen H Lockhart, Joseph Wilcox, Kelly Smits, Joan Etzell, Sami Albeiroti, Michele DeRee, Christine Flaherty, Sheila Genolaga, Michelle Goodreau, Farah Refai, Alexandra Restall, Katarina Lanner-Cusin and Kristen MJ Azar in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455065211063300 for COVID-19 in pregnancy by race and ethnicity: Implications for development of a vaccination strategy by Alice Pressman, Stephen H Lockhart, Joseph Wilcox, Kelly Smits, Joan Etzell, Sami Albeiroti, Michele DeRee, Christine Flaherty, Sheila Genolaga, Michelle Goodreau, Farah Refai, Alexandra Restall, Katarina Lanner-Cusin and Kristen MJ Azar in Women’s Health
Acknowledgments
The authors wish to thank the David and Lucile Packard Foundation for their generous grant to support this research. The research presented in this manuscript has been accepted for presentation at the American Public Health Association annual meeting in Denver, Colorado, in October 2021.
Footnotes
Author contributions: Both A.P. and S.H.L. contributed to the conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, supervision, validation, visualization, writing-original draft, and writing-review & editing. In addition, A.P. contributed to project administration. J.W. contributed to data curation, formal analysis, methodology, validation, and writing-review & editing. K.S. contributed to funding acquisition, investigation, methodology, project administration, visualization, writing-original draft, and writing-review & editing. J.E. and C.F. contributed to methodology and writing-review & editing. S.A. contributed to data curation, investigation, methodology, and writing-review & editing. M.D. contributed to data curation, investigation, methodology, project administration, supervision, and writing-review & editing. S.G, M.G., F.R, and A.R, contributed to data curation, methodology, project administration, and writing-review & editing. In addition, S.G. contributed to supervision. K.L-C contributed to conceptualization, investigation, methodology, writing-original draft, and writing-review & editing. K.M.J.A contributed to investigation, methodology, supervision, and writing-review & editing.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.H.L. is on the boards of the ECRI Institute, the E.O. Wilson Biodiversity Foundation, the David and Lucile Packard Foundation, NRC Health, Molina Healthcare, and Parks California and is a retired employee of Sutter Health. K.L.-C. is a practicing Obstetrician and Gynecologist for the Sutter East Bay Medical Foundation. All other authors are employees of and receive salary from Sutter Health and have no other conflicts to report.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the David and Lucile Packard Foundation. The sponsor had no involvement in any of the scientific research or publication. Salary support for the authors was provided by Sutter Health.
Informed consent waiver: Informed consent was waived pursuant to 45CFR46.116(f)(3). This waiver was reviewed by the institutional review board (IRB) using the full Board review procedure as described by the Common Rule. This approval was based on IRB determinations that there is an adequate plan to protect the patient identifiers from improper use and disclosure; there is an adequate plan to destroy the identifiers at the earliest opportunity unless the retention is required by law; there are adequate written assurances that the PHI will not be reused or disclosed to any person or entity not covered by this Waiver, unless required by law for authorized oversight of the research project; there is adequate justification that the PHI is critical to the conduct of the research project and that the research could not be conducted by obtaining patient authorization.
ORCID iDs: Alice Pressman  https://orcid.org/0000-0003-2775-3275
https://orcid.org/0000-0003-2775-3275
Stephen H Lockhart  https://orcid.org/0000-0002-1211-3817
https://orcid.org/0000-0002-1211-3817
Kelly Smits  https://orcid.org/0000-0001-8686-4173
https://orcid.org/0000-0001-8686-4173
Kristen MJ Azar  https://orcid.org/0000-0003-2801-3156
https://orcid.org/0000-0003-2801-3156
Sharing of research materials: Please email corresponding author for more information or assistance with applying the CVEI or any statistical models presented in this manuscript.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014; 384(9947): 980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Racial and ethnic disparities continue in pregnancy-related deaths. https://www.cdc.gov/media/releases/2019/p0905-racial-ethnic-disparities-pregnancy-deaths.html
- 3. Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html
- 4. Chen J, Cox S, Kuklina EV, et al. Assessment of incidence and factors associated with severe maternal morbidity after delivery discharge among women in the US. JAMA Netw Open 2021; 4(2): e2036148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bassett MT, Chen JT, Krieger N. Variation in racial/ethnic disparities in COVID-19 mortality by age in the United States: a cross-sectional study. PLoS Med 2020; 17(10): e1003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Azar KMJ, Shen Z, Romanelli RJ, et al. Disparities in outcomes among COVID-19 patients in a large health care system in California. Health Aff (Millwood) 2020; 39(7): 1253–1262. [DOI] [PubMed] [Google Scholar]
- 7. Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020; 323(24): 2466–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yancy CW. COVID-19 and African Americans. JAMA 2020; 323(19): 1891–1892. [DOI] [PubMed] [Google Scholar]
- 9. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory—confirmed Coronavirus Disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69(15): 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El Chaar M, King K, Galvez Lima A. Are black and Hispanic persons disproportionately affected by COVID-19 because of higher obesity rates. Surg Obes Relat Dis 2020; 16(8): 1096–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore KM, Suthar MS. Comprehensive analysis of COVID-19 during pregnancy. Biochem Biophys Res Commun 2021; 538: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. APM Research Lab. The color of coronavirus: COVID-19 deaths by race and ethnicity in the U.S. https://www.apmresearchlab.org/covid/deaths-by-race
- 13. Azar KMJ, Lockhart SH, Shen Z, et al. Disparities among racially/ethnically marginalized groups in the COVID-19 pandemic persist regardless of statewide shelter-in-place policies: an analysis from Northern California. Am J Epidemiol 2021; 190(11): 2300–2313. [DOI] [PubMed] [Google Scholar]
- 14. Mullins E, Evans D, Viner RM, et al. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol 2020; 55(5): 586–592. [DOI] [PubMed] [Google Scholar]
- 15. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021; 175(8): 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand 2020; 99(7): 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vouga M, Favre G, Martinez-Perez O, et al. Maternal outcomes and risk factors for COVID-19 severity among pregnant women. Sci Rep 2021; 11(1): 13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karasek D, Baer R, McLemore M. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Health Am 2021; 2: 100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sutter Health. About Sutter Health. https://www.sutterhealth.org/about
- 20. Centers for Disease Control Prevention. COVID data tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 21. Shimabukuro TT, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med 2021; 384(24): 2273–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pressman AR, Lockhart SH, Shen Z, et al. Measuring and promoting SARS-CoV-2 Vaccine Equity: development of a COVID-19 Vaccine Equity Index. Health Equity 2021; 5(1): 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sutter Health. What is Sutter Health? https://www.sutterhealth.org/about/what-is-sutter-health
- 24. Sutter Health. Keeping our Care Connected. [Google Scholar]
- 25. Qu J, Wu C, Li X, et al. Profile of immunoglobulin G and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis 2020; 71(16): 2255–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wölfel R, Corman VM, Guggemos W, et al. Author Correction: Virological assessment of hospitalized patients with COVID-2019. Nature 2020; 588(7839): E35. [DOI] [PubMed] [Google Scholar]
- 27. Centers for Disease Control Prevention. Interim guidelines for COVID-19 antibody testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html
- 28. Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med 2021; 27(10): 1693–1695. [DOI] [PubMed] [Google Scholar]
- 29. Kwok KO, Lai F, Wei WI, et al. Herd immunity—estimating the level required to halt the COVID-19 epidemics in affected countries. J Infect 2020; 80(6): e32–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med 2021; 385(7): 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris RJ, Hall JA, Zaidi A, et al. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med 2021; 385(8): 759–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Society for Maternal-Fetal Medicine. Provider considerations for engaging in COVID vaccination considerations. Washington, DC: Society for Maternal-Fetal Medicine, 2020. [Google Scholar]
- 33. Liu Y, Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med 2021; 28(7): taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Little RJA, Rubin DB. Statistical analysis with missing data. 2nd ed. Hoboken, NJ: Wiley, 2002. [Google Scholar]
- 35. Office of the Assistant Secretary for Planning Evaluation. HHS and implementation guidance on data collection standards for race ethnicity sex primary language disability status. Washington, DC: Office of the Assistant Secretary for Planning Evaluation, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-whe-10.1177_17455065211063300 for COVID-19 in pregnancy by race and ethnicity: Implications for development of a vaccination strategy by Alice Pressman, Stephen H Lockhart, Joseph Wilcox, Kelly Smits, Joan Etzell, Sami Albeiroti, Michele DeRee, Christine Flaherty, Sheila Genolaga, Michelle Goodreau, Farah Refai, Alexandra Restall, Katarina Lanner-Cusin and Kristen MJ Azar in Women’s Health
Supplemental material, sj-docx-2-whe-10.1177_17455065211063300 for COVID-19 in pregnancy by race and ethnicity: Implications for development of a vaccination strategy by Alice Pressman, Stephen H Lockhart, Joseph Wilcox, Kelly Smits, Joan Etzell, Sami Albeiroti, Michele DeRee, Christine Flaherty, Sheila Genolaga, Michelle Goodreau, Farah Refai, Alexandra Restall, Katarina Lanner-Cusin and Kristen MJ Azar in Women’s Health