Abstract
Eltrombopag is a small molecule, thrombopoietin receptor agonist approved for the treatment of patients with aplastic anemia and chronic immune thrombocytopenia. It is also a polyvalent cation chelator and inhibits leukemia cell proliferation via reduction of intracellular iron. The in vivo efficacy of eltrombopag was tested against a panel of six Pediatric Preclinical Testing Consortium osteosarcoma xenografts at doses of 5 mg/kg/day (moderate dose) and 50 mg/kg/day (high dose). Eltrombopag, at moderate doses, failed to significantly improve event-free survival (EFS) in 6/6 models. At high doses, eltrombopag significantly prolonged EFS in 2/2 models, though the effect size was small. All models tested demonstrated progressive disease. While eltrombopag did not meaningfully inhibit osteosarcoma growth, it also did not stimulate tumor growth, suggesting it may be safely investigated as a supportive care agent to enhance platelet recovery post chemotherapy.
Keywords: Eltrombopag, osteosarcoma, patient derived xenograft
Introduction
Osteosarcoma is the most common primary malignancy of bone in children and adolescents.1 Survival rates for patients with osteosarcoma have not meaningfully changed since the recognition of the efficacy of combination chemotherapy in the 1980s. Neoadjuvant and adjuvant chemotherapy, typically with a regimen of high-dose methotrexate, doxorubicin, and cisplatin, combined with surgical resection of the gross disease, still remains the most common treatment. Patients with localized osteosarcoma experience a five-year event-free survival (EFS) rate of approximately 70% while those with metastatic or recurrent disease have an overall survival rate less than 30%.2,3 Given these poor prognoses, novels agents are needed to improve outcomes for patients diagnosed with osteosarcoma.
Eltrombopag (EP), a small molecule thrombopoietin receptor (TPO-R) agonist, is used in the treatment of chronic immune thrombocytopenia purpura (ITP) and is approved by the U.S. Food and Drug Administration for the treatment of severe aplastic anemia.4,5 EP has also been studied for the prophylaxis and or treatment of myelosuppression secondary to chemotherapy.6 Unlike prior platelet agonists, EP is not immunogenic, and thus does not lead to antibody formation.7 Prior in vitro and in vivo studies in acute myeloid leukemia (AML) have demonstrated that EP has antitumor activity against cancer cells that is independent of TPO-R binding. EP is a polyvalent cation that reduces leukemia cell division via depletion of intracellular iron levels and the inactivation of the rate-determining mitotic enzyme ribonucleotide reductase.8–10 Similarly, in vitro studies for osteosarcoma demonstrated EP inhibited cell growth in a dose dependent manner (Personal Communication, L Gennarini). These studies, which have not yet been published, demonstrated that EP treatment leads to a decreased in intracellular iron and upregulation of the transferrin receptor, the iron transporter. In addition, preloading the cells with iron prior to treatment with EP led to significant rescue from the anti-proliferative effects of EP, suggesting osteosarcoma cells have some level of dependence on iron for growth. The current study assessed the potential anti-cancer efficacy of EP against the Pediatric Preclinical Testing Consortium (PPTC) in vivo osteosarcoma models. Given the potential use of eltrombopag as a supportive care agent for patients with chemotherapy-induced thrombocytopenia, evaluation of eltrombopag was prioritized over other iron chelators.
Materials and methods
Osteosarcoma patient derived xenograft (PDX) models (OS2, OS9, OS31, OS33, OS36, and OS60) were heterotopically implanted into the flanks of 4–6 week CB17SC scid−/− female mice (Taconic Farms, Germantown, NY). These models were initially established by surgically inserting minced fresh tumor specimens, obtained after pathology review, subcutaneously into the flanks of CB17SC scid−/− mice. PDX models OS2, OS9, OS31, and OS 33 originated from primary tumors located in the bone, without prior exposure to chemotherapy. OS36 originated from a lung metastasis exposed to prior treatment and OS60 originated from a bone relapse and was also exposed to prior treatment. All pdx models have been genomically characterized.11 As per prior PPTC studies, treatment was initiated when tumors were between 5–7mm and mice were sacrificed when tumors grew to 4 times their initial size.12 EP (Novartis, Basel, Switzerland) was administered at a dose of 5 mg/kg/day five days per week to all six PDX models via oral gavage with planned administration for four weeks. High dose EP (50 mg/kg/day) was administered to two models, selected at random, (OS2, OS9) per the same schedule. In prior studies EP was administered at doses between 5 mg/kg/day and 50 mg/kg/day with adequate safety, and a dose of 5 mg/kg/day inhibited leukemia cell growth in AML mouse models.9,13 Since EP stimulates platelet production via binding to the TPO-R, we wanted to determine whether there was any relationship between TPO-R expression and inhibition of osteosarcoma growth. The PDX models exhibited limited or no MPL (thrombopoietin receptor) RNA expression as determined via RNA-Seq. Tumor volumes were measured as previously described and a control cohort of mice that was administered vehicle was utilized for each PDX model.12 During testing, all mice were maintained under barrier conditions, and experiments were conducted using protocols approved by the Institutional Animal Care and Use Committees at MD Anderson Cancer Center and the Albert Einstein College of Medicine. EP’s activity was evaluated using standard PPTC measures, including time to event (EFS T/C), tumor growth delay (tumor volume T/C), and objective responses as previously described.12 The Gehan-Wilcoxon test (survival package for R) was used to compare EFS distributions between treatment and control groups. P-values were two-sided and were considered statistically significant if <0.05.
Results
Eltrombopag at a dose of 5 mg/kg/day was tested against six PPTC osteosarcoma xenografts. All mice tolerated therapy, there were no significant changes in weight in the treated mice, and there were no toxic deaths during the study period. EP did not significantly prolong time to event in any of the six models tested (Figure 1 and Table 1). One of the models tested (OS31) demonstrated a significantly smaller relative average minimum relative tumor volume compared to control, however, this difference was small and reflected an increase in tumor volume in treated animals compared to baseline. No objective responses were observed, and all models met criteria for progressive disease (PD1) (Table 1). EP did not significantly enhance tumor growth in any of the models tested.
Figure 1.
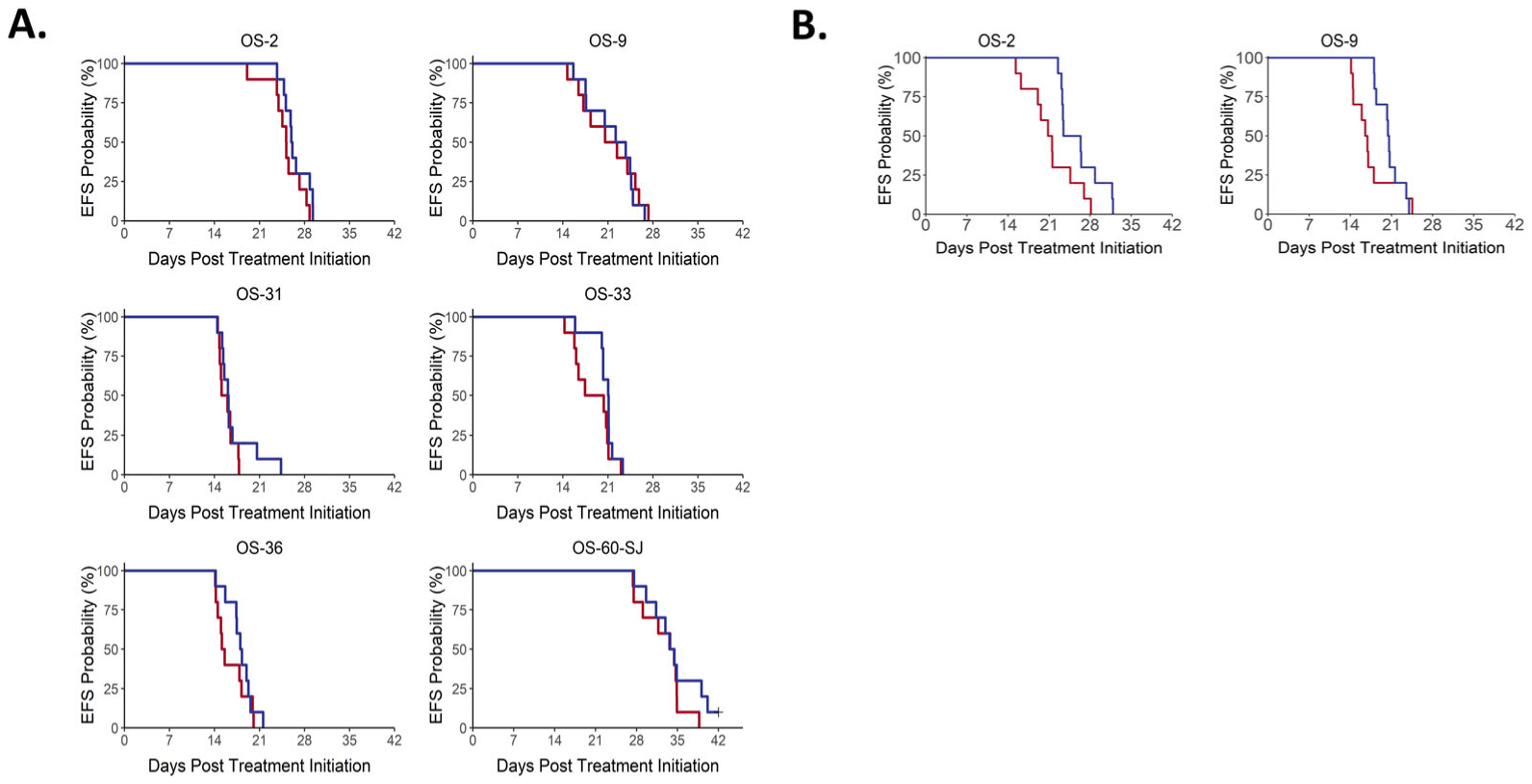
Event-free survival to eltrombopag A. (5 mg/kg) and B. (50 mg/kg) across all PDX models. No significant differences in time to event were observed in mice treated with 5 mg/kg/day. Both PDX models treated with 50 mg/kg/day experienced significant prolongation in time to event.
Table 1.
Osteosarcoma PDX model response to eltrombopag.
| Model | aKM med (days) | bEFS T - C (days) | cEFS T/C | dp-value Gehan-Wilcoxon | eminRTV mean ± SD | fminRTV p-value | Objective Response Measure |
|---|---|---|---|---|---|---|---|
| 5 mg/kg | |||||||
| OS-2 | 26.0 | 0.9 | 1.03 | p = 0.172 | 1.134 ± 0.091 | p = 0.631 | PD1 |
| OS-9 | 23.0 | 1.5 | 1.07 | p = 0.881 | 1.686 ± 0.461 | p = 0.739 | PD1 |
| OS-31 | 16.1 | 0.6 | 1.04 | p = 0.407 | 1.774 ± 0.188 | p = 0.011 | PD1 |
| OS-33 | 21.1 | 2.2 | 1.12 | p = 0.109 | 1.472 ± 0.236 | p = 0.123 | PD1 |
| OS-36 | 18.1 | 2.8 | 1.18 | p = 0.170 | 2.019 ± 0.352 | p = 0.684 | PD1 |
| OS-60-SJ | 34.1 | 0.0 | 1.00 | p = 0.452 | 1.228 ± 0.113 | p = 0.353 | PD1 |
| 50 mg/kg | |||||||
| OS-2 | 24.9 | 3.7 | 1.2 | p = 0.015 | 1.403 ± 0.241 | p = 0.529 | PD1 |
| OS-9 | 20.5 | 3.8 | 1.2 | p = 0.014 | 1.752 ± 0.197 | p = 0.143 | PD1 |
Kaplan–Meier estimate of median days to event;
EFS (event-free survival) T-C is the median time to event of the treated group minus the median time to event of the control groups;
EFS (event-free survival) T/C is the ratio of the median time to event between treated and control groups;
P-values comparing event-free survival between the treated and control groups;
minRTV (minimum relative tumor volume);
P-values comparing the minRTV between the treated and control groups, P-value <0.05 is considered statistically significant.
High dose EP (50 mg/kg/day) was tested in two PDX models, OS2 and OS9, and resulted in a small, yet statistically significant, prolongation in time to event in both models (p = 0.015 and p = 0.014, respectively). The ratio for the median time to event for the treated versus control animals (EFS T/C) was 1.2 for each model. No objective responses were seen in either model, with both meeting criteria for progressive disease (PD1).
Discussion
Eltrombopag is effective in treating thrombocytopenia in patients with chronic ITP, aplastic anemia, AML and myelodysplastic syndrome (MDS), and chemotherapy-induced myelosuppression.4,6,14,15 Recent studies in hematologic malignancies have demonstrated EP exerts an anti-proliferative effect, raising the possibility that the supportive care agent potentially could be utilized to both treat chemotherapy-induced thrombocytopenia as well as inhibit cancer growth. The current study sought to assess the anti-cancer efficacy of eltrombopag in osteosarcoma and found that it had limited single agent activity.
Structurally, EP is a polyvalent cation chelator, and it inhibits leukemia cell proliferation and cell cycling via reduction of intracellular iron levels.9 Iron depletion results in the inactivation of ribonucleotide reductase, the rate-determining enzyme of mitosis; as a result, iron chelators are attractive potential antitumor agents.8,16 Prior studies demonstrated that EP’s effect on leukemia cells is independent of TPO-R expression. EP enters cells rapidly via its low molecular weight and high lipophilicity, and very effectively chelates iron found in the intracellular labile iron pool.9,17 The resulting depletion of intracellular iron causes a block in cell division through the inhibition of cyclin-dependent kinases and ribonucleotide reductase. Myeloid leukemia cells, given their dependence on intracellular iron, are highly sensitive to EP treatment.9 Despite the interesting preclinical rationale for eltrombopag for myeloid neoplasms, randomized trials evaluating eltrombopag for patients with AML and/or MDS have failed to show significant clinical anti-cancer benefit for its use.18,19
In this study, we found that EP does not exhibit the same antitumor activity on osteosarcoma cells that were observed against AML and MDS samples. Prior in vitro studies demonstrated EP inhibited osteosarcoma cell growth in a dose dependent manner (Personal Communication, L Gennarini). However, EP at a dose of 5 mg/kg/day failed to prolonged EFS in any of the models tested, and high dose EP led to only small differences in event free survival. EP’s inability to effectively inhibit osteosarcoma tumor growth in in vivo models may be due to different dependencies on intracellular cations across cancer types. In vitro, EP depletes intracellular iron in osteosarcoma cells, however, repletion of iron, unlike in AML cell, only partially rescues the osteosarcoma cells. Further studies are needed to determine the extent to which EP chelates polyvalent other polyvalent cations such as calcium, copper, and magnesium in osteosarcoma cells and the effect of depletion of these cations on osteosarcoma cell growth.
Despite the lack of anti-cancer activity of single agent EP against osteosarcoma cells, EP could potentially be considered as a potential supportive care agent for patients with osteosarcoma. Standard chemotherapy for osteosarcoma is highly myelosuppressive and leads to prolonged thrombocytopenia that can result in delays in treatment. As EP did not stimulate osteosarcoma growth in any of the models tested, EP may be able to be utilized to promote platelet production after the administration of chemotherapy, similar to the use of granulocyte colony stimulating factor to promote neutrophil production. Clinical evaluations would be needed to determine whether patients with osteosarcoma benefit from the use of EP to reduce the effects of chemotherapy-induced thrombocytopenia.
Funding
This work was supported by Grants CA199221 and CA199222 from the National Cancer Institute, Swim Across America, and the Foster Foundation. Eltrombopag was supplied by Novartis.
Abbreviations:
- EFS
Event free survival
- EP
Eltrombopag
- TPO-R
Thrombopoietin receptor
- ITP
Immune thrombocytopenic purpura
- AML
Acute myeloid leukemia
- PPTC
Pediatric Preclinical Testing Consortium
- PDX
Patient derived xenograft
- PD1
Progressive disease
- MDS
Myelodysplastic syndrome
Footnotes
Disclosure statement
The authors do not report any conflicts of interest.
References
- 1.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40(4):523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18(1):39–50. doi: 10.1080/14737140.2018.1413939. [DOI] [PubMed] [Google Scholar]
- 3.Meyers PA, Gorlick R. Osteosarcoma. Pediatr Clin North Am. 1997;44(4):973–989. doi: 10.1016/s0031-3955(05)70540-x. [DOI] [PubMed] [Google Scholar]
- 4.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–2247. doi: 10.1056/NEJMoa073275. [DOI] [PubMed] [Google Scholar]
- 5.Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–1656. doi: 10.1056/NEJMra1413485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winer ES, Safran H, Karaszewska B, et al. Eltrombopag for thrombocytopenia in patients with advanced solid tumors receiving gemcitabine-based chemotherapy: a randomized, placebo-controlled phase 2 study. Int J Hematol. 2017;106(6):765–776. doi: 10.1007/s12185-017-2319-9. [DOI] [PubMed] [Google Scholar]
- 7.Basciano PA, Bussel JB. Thrombopoietin-receptor agonists. Curr Opin Hematol. 2012;19(5): 392–398. [DOI] [PubMed] [Google Scholar]
- 8.Richardson D, Kalinowski D, Lau S, Jansson P, Lovejoy D. Cancer cell iron metabolism and the development of potent iron chelators as anti-tumour agents. Biochim Biophys Acta. 2009;1790(7):702–717. doi: 10.1016/j.bbagen.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012;120(2): 386–394. blood-2011-2012-399667. doi: 10.1182/blood-2011-12-399667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Will B, Kawahara M, Luciano JP, et al. Effect of the nonpeptide thrombopoietin receptor agonist Eltrombopag on bone marrow cells from patients with acute myeloid leukemia and myelodysplastic syndrome. Blood. 2009;114(18):3899–3908. doi: 10.1182/blood-2009-04-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rokita JL, Rathi KS, Cardenas MF, et al. Genomic profiling of childhood tumor patient-derived xenograft models to enable rational clinical trial design. Cell Rep. 2019;29(6): 1675–1689. e1679. doi: 10.1016/j.celrep.2019.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49(7):928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Tsai Y, Nowak I, Liesveld J, Chen Y. Eltrombopag, a thrombopoietin receptor agonist, enhances human umbilical cord blood hematopoietic stem/primitive progenitor cell expansion and promotes multi-lineage hematopoiesis. Stem Cell Res. 2012;9(2):77–86. doi: 10.1016/j.scr.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavroudi I, Pyrovolaki K, Pavlaki K, et al. Effect of the nonpeptide thrombopoietin receptor agonist eltrombopag on megakaryopoiesis of patients with lower risk myelodysplastic syndrome. Leuk Res. 2011;35(3):323–328. doi: 10.1016/j.leukres.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pahl PM, Horwitz LD. Cell permeable iron chelators as potential cancer chemotherapeutic agents. Cancer Invest. 2005;23(8):683–691. doi: 10.1080/07357900500359976. [DOI] [PubMed] [Google Scholar]
- 17.Vlachodimitropoulou E, Chen Y-L, Garbowski M, et al. Eltrombopag: a powerful chelator of cellular or extracellular iron (III) alone or combined with a second chelator. Blood. 2017; 130(17):1923–1933. blood-2016-2010-740241. doi: 10.1182/blood-2016-10-740241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickinson M, Cherif H, Fenaux P, et al. Azacitidine with or without eltrombopag for first-line treatment of intermediate- or high-risk MDS with thrombocytopenia. Blood. 2018; 132(25):2629–2638. doi: 10.1182/blood-2018-06-855221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey N, Jang JH, Szer J, et al. Eltrombopag treatment during induction chemotherapy for acute myeloid leukaemia: a randomised, double-blind, phase 2 study. Lancet Haematol. 2019;6(3):e122–e131. doi: 10.1016/S2352-3026(18)30231-X. [DOI] [PubMed] [Google Scholar]


