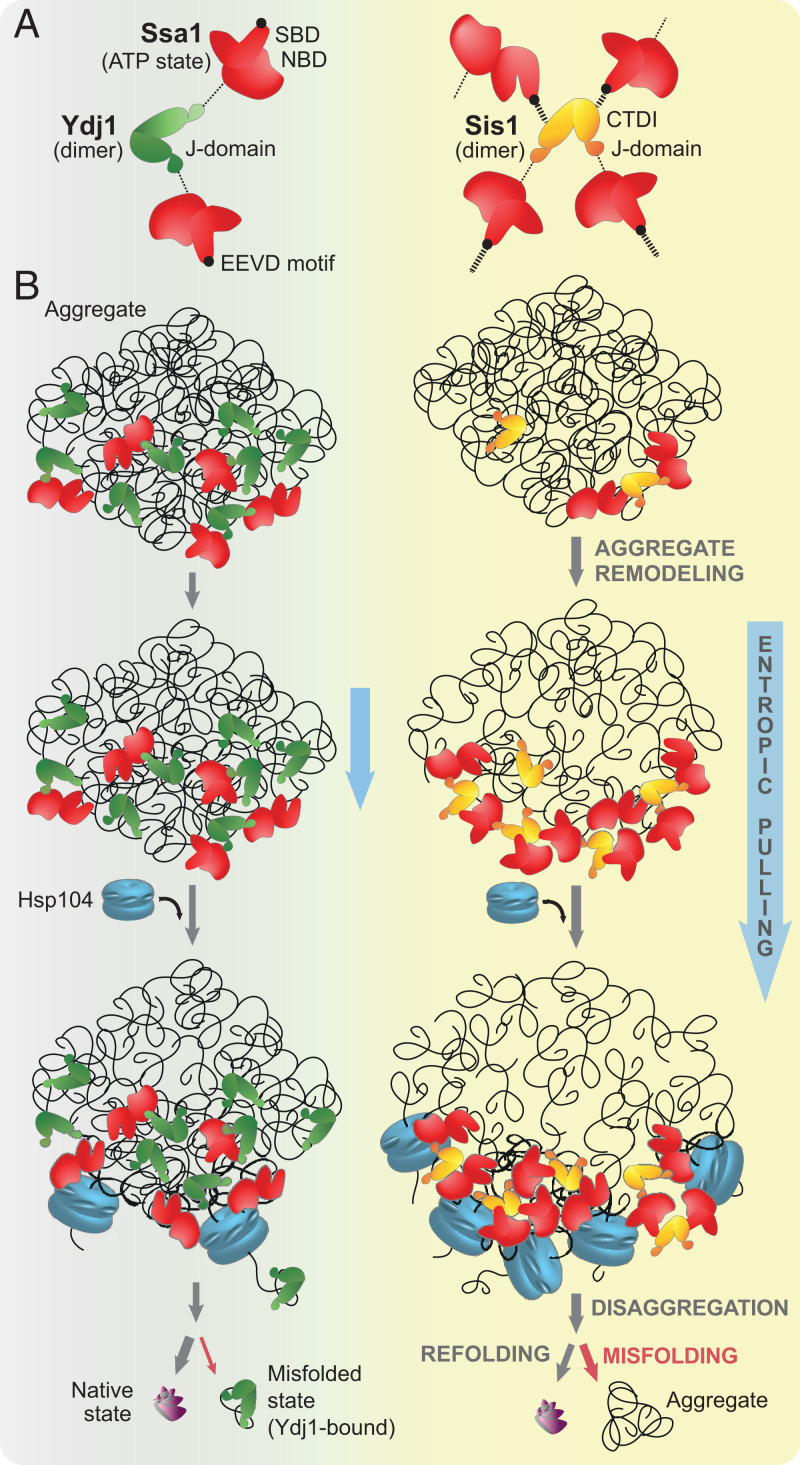
Fig. 5.
Model of distinctive contribution of Class A and B JDPs to protein disaggregation. (A) While both Ydj1 and Sis1 bind and stimulate Hsp70 through the J-domain binding to Ssa1 NBD, in Sis1, the additional interaction with the EEVD motif expands the network of possible interactions. (B) Despite the initially a lower level of aggregate binding, with time, Sis1 enables more abundant loading of Ssa1 molecules on the aggregated substrate. Larger chaperone complexes likely potentiate entropic pulling (54), which remodels aggregates in a way that favors more abundant chaperone binding. Saturation of protein aggregate with Hsp70 enhances Hsp104 docking and disaggregation. Disaggregated polypeptides with misfolding proclivity are better substrates for Ydj1 than Sis1 (26). Ydj1 binding prevents their aggregation, which promotes their return on correct folding pathways.