Significance
Tropical forests disappear rapidly through deforestation but also have the potential to regrow naturally through a process called secondary succession. To advance successional theory, it is essential to understand how these secondary forests and their assembly vary across broad spatial scales. We do so by synthesizing continental-scale patterns in succession using a functional trait approach. We show that the start and pathway of succession varies with climatic water availability. In dry forests, succession is driven by drought tolerance traits and in wet forests by shade tolerance traits. Based on these successional principles, we propose an ecologically sound strategy to improve active forest restoration.
Keywords: tropical forest, secondary succession, functional traits, rainfall, community assembly
Abstract
One-third of all Neotropical forests are secondary forests that regrow naturally after agricultural use through secondary succession. We need to understand better how and why succession varies across environmental gradients and broad geographic scales. Here, we analyze functional recovery using community data on seven plant characteristics (traits) of 1,016 forest plots from 30 chronosequence sites across the Neotropics. By analyzing communities in terms of their traits, we enhance understanding of the mechanisms of succession, assess ecosystem recovery, and use these insights to propose successful forest restoration strategies. Wet and dry forests diverged markedly for several traits that increase growth rate in wet forests but come at the expense of reduced drought tolerance, delay, or avoidance, which is important in seasonally dry forests. Dry and wet forests showed different successional pathways for several traits. In dry forests, species turnover is driven by drought tolerance traits that are important early in succession and in wet forests by shade tolerance traits that are important later in succession. In both forests, deciduous and compound-leaved trees decreased with forest age, probably because microclimatic conditions became less hot and dry. Our results suggest that climatic water availability drives functional recovery by influencing the start and trajectory of succession, resulting in a convergence of community trait values with forest age when vegetation cover builds up. Within plots, the range in functional trait values increased with age. Based on the observed successional trait changes, we indicate the consequences for carbon and nutrient cycling and propose an ecologically sound strategy to improve forest restoration success.
Tropical forests are globally important for biodiversity conservation and carbon and water cycling but are converted at an alarming rate for agricultural use and pastureland. After agricultural abandonment, forests may regrow naturally through secondary succession. These regrowing secondary forests comprise as much as a third of the Neotropical forest area (1). To advance successional theory and to design successful site-specific forest restoration strategies, it is essential to understand how secondary forests and their community assembly vary across broad spatial scales (2). This knowledge is urgent, given global commitments to reforest 3.5 million km2 by 2030 (3) for restoring biodiversity and site productivity and to capitalize on the climate change mitigation potential of regrowing forests (1). Most of this restoration is likely to be achieved through passive restoration by natural regrowth, but where needed, it can be achieved through active restoration such as tree planting (1, 4).
Although the study of succession and community assembly has a long history (5, 6), it is difficult to synthesize results and draw generalizations because biogeographically distinct areas differ strikingly in species composition. Analyzing community composition based on their species-specific traits allows to quantitatively compare species using the same ecological yardstick and synthesize data across continental scales. More importantly, traits influence species performance and, hence, community assembly and ecosystem processes (7). By analyzing communities in terms of their functional traits, we can gain a deeper understanding of the mechanisms of succession and recovery of ecosystem functions over time.
Community assembly is driven by a hierarchical set of drivers that operate from a broad regional scale (e.g., climate and soil that determine resource availability) to the landscape scale (e.g., surrounding forest cover that determines seed availability) to the local scale (e.g., previous land use intensity and current forest use that leave legacies in the vegetation and soils) (8). At the local scale, light availability declines as succession proceeds, whereas changes in water and nutrient resources are more variable (9). Successional change is thought to be partly governed by a trade-off between resource acquisition and conservation across species (10, 11) in which early succession favors species with traits for fast resource acquisition, and later succession favors species with traits for resource conservation. Forest successional theory predicts therefore that light-demanding early successional species geared toward resource acquisition and growth (i.e., acquisitive species) are replaced by shade-tolerant late successional species geared toward resource conservation and persistence (i.e., conservative species) (7, 10, 12). However, successional pathways may vary across large-scale environmental gradients depending on the traits of the regional species pool and how biotic and abiotic conditions change during succession (13). Across lowland tropical forests, rainfall and soil fertility are thought to be the two most important gradients shaping forest composition and functioning (14). At the start of succession, dry and wet forests differ markedly in macroclimate, drought, and heat stress (15), which may select for species with different trait values. Later in succession, however, environmental conditions in the understory of dry and wet forests become more similar (shaded, cooler, and humid) (16), which may select for regenerating tree species with similar trait values (13). In a recent Neotropical-wide analysis, Poorter et al. (17) found that in wet forests, community average wood density (WD) values indeed proceeded from soft-wooded, fast-growing acquisitive species early in succession toward hard-wooded, slow-growing conservative species later in succession. However, in dry forests, succession proceeded from high to low WD (i.e., conservative to acquisitive trait values), probably because early in succession, harsh climatic conditions and strong edaphic and atmospheric drought select for dense-wooded species with high cavitation resistance, whereas later in succession, more benign, cool, and humid understory conditions allow for the establishment of soft-wooded species with lower cavitation resistance. Combined, these processes led to dry and wet forests having increasingly similar WD values (i.e., “convergence”) over time.
The question remains whether these findings for a stem trait can be extended to leaf traits, as stem and leaves fulfill different functions, have different longevity, and may be part of different trait syndromes (18). Stems are important for biomechanical and hydraulic support and might therefore be under stronger selection by soil water status and wind, whereas leaves are important for gas and heat exchange and carbon gain and may therefore be under stronger selection of irradiance and atmospheric drought (i.e., high vapor pressure deficit). Stems are long-lived and cannot be replaced, which may imply that they are exposed to stronger selection filters, whereas leaves are short lived and can be easily replaced, which may imply that they are under weaker selection filters and may show a larger variety of strategies. Finally, it is unclear whether stem and leaves are part of the same trait syndrome, as some studies show that stem and leaf traits are strongly associated (19), whereas others show that they can vary independently (18).
In addition to macroclimate, the landscape context and previous land use can also affect community assembly in which increased land use intensity and landscape fragmentation lead to shorter statured communities with smaller seeds and conservative trait values in the Sahel (20). In contrast, land use intensity led to more acquisitive trait values in the Brazilian Caatinga (21).
During succession, communities not only show a shift in average trait values but also in the range of trait values. In general, the trait range may be narrow early in succession because of strong environmental filtering, whereas later in succession, the trait range may increase (22) because of an accumulation of new species with different trait values, the persistence of long-lived pioneers with early successional trait values during succession, and competitive interactions that favor limiting similarity and, hence, trait divergence of cooccurring species (23). Thus far, it is not clear how dry and wet forests differ in trait variation during succession. Trait variation at the start of succession may be smaller for dry compared to wet forests due to stronger environmental filtering as a result of harsher climatic conditions. Trait variation may increase more rapidly during succession for wet compared to dry forests as a result of a more rapid increase in species richness and because of a taller forest with longer and steeper environmental gradients, offering a wider range of potential niches (13).
To date, no study has evaluated functional recovery and underlying environmental drivers for a suite of leaf traits in a systematic way across broad geographic scales. Here, we assess how community trait means and variation recover during succession and how this functional recovery is predicted by variation in rainfall, soil fertility, surrounding forest cover, and previous land use. Across these broad geographic scales, macroclimate is probably the strongest predictor of community assembly and therefore the main focus of our hypotheses. We hypothesize that 1) dry and wet forests show contrasting successional pathways with initial large trait differences between dry and wet forests because of different abiotic conditions at the start of succession and convergence of trait values over time because abiotic and biotic conditions become more similar, and 2) trait variation within a patch increases during succession, more strongly in wet compared to dry forests, because over time more species with different trait values arrive.
Methods
We analyze functional recovery at a broad spatial scale using original data from 30 sites, 1,016 plots, and >127 thousand trees, covering most of the latitudinal, climatic, and soil fertility gradients in the lowland Neotropics (SI Appendix, Fig. S1 and Table S1). Ideally, succession is monitored over time, but this has been done for only very few sites and for a rather short period (up to 20 y maximum). To provide a long-term perspective on succession, we use chronosequences in which plots that differ in time since agricultural abandonment (1 up to 100 y) are compared. We focus on seven traits related to resource use and adaptations to abiotic conditions: leaf deciduousness ((brevi)deciduous versus evergreen species), leaf compoundness (compound versus simple leaves), leaf size (LS), specific leaf area (SLA; leaf area per unit leaf mass), leaf nitrogen concentration (LNC), and nitrogen-fixing ability (NF). These are important traits that shape plant responses to the environment (SI Appendix, Table S2) and also impact the recovery of forest ecosystem functions such as carbon, water, and nutrient cycling. We also included WD to evaluate whether leaf traits show similar successional pathways as this stem trait. For each plot, community trait averages (the community-weighted mean; CWM) and trait variation (the trait range, calculated as the trait value of the 90th percentile minus the trait value of the 10th percentile) were calculated based on the trait values and weighted by the basal area of the species in a plot. For each site, functional recovery was analyzed by regressing community trait values against ln(forest age). In addition, we used linear mixed models to assess how CWM trait values and trait ranges were affected by a core model consisting of age, climatic water availability (CWA), and their interaction and potentially by soil cation exchange capacity (CEC; an indicator of soil fertility), surrounding forest cover, previous land use (agriculture versus pastures), and their interactions with forest age.
Results
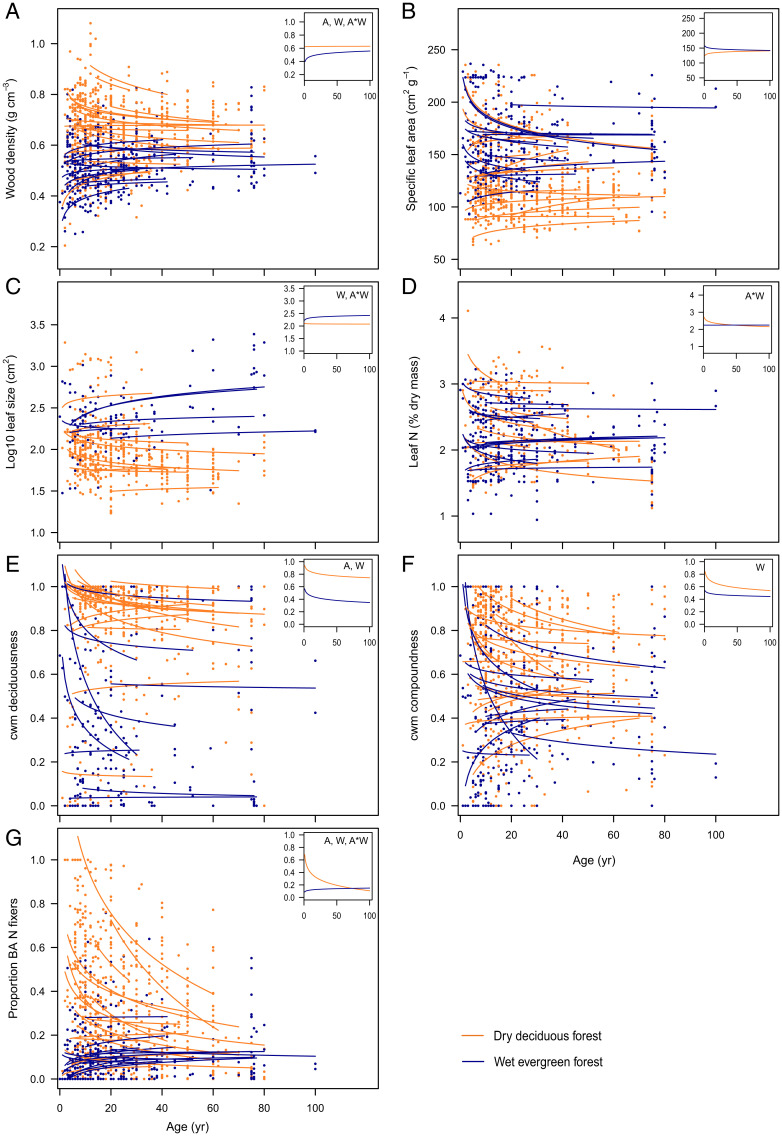
Across all plots, all CWM traits except deciduousness showed a funnel-shaped relationship with time (as evidenced by quantile regressions; Table 1), with wide variation between sites and plots for trait CWM at the start of succession and smaller trait variation later in succession (Fig. 1). Only LS showed a reversed funnel with time. These funnel-shaped relationships were partly caused by site-specific differences in the start (i.e., at 5 y, when an initial vegetation has developed) and the trajectory (i.e., slope) of succession. Predicted CWM traits at 5 y varied strongly across sites; WD varied 3.1-fold (from 0.37 to 1.14 g ⋅ cm−3), leaf deciduousness varied from 0 to 100%, compoundness from 7 to 100%, LS varied 74-fold (5.7 to 421 cm2), SLA 3.7-fold (56 to 206 cm2 ⋅ g−1), LNC 2.7-fold (1.5 to 3.9%), and the proportion of NF trees varied from 0.02 to 1.
Table 1.
Results of a quantile regression on the 10th percentile and 90th percentile of the CWM trait values against ln(forest age)
| Trait | 10th percentile | 90th percentile | Funnel | ||
| Intercept | Slope | Intercept | Slope | ||
| WD | 0.434 | 0.025 | 0.773 | −0.007 | Y |
| SLA | 93.889 | 0.240 | 193.120 | −12.021 | Y |
| LS | 1.646 | −0.054 | 2.516 | 0.010 | Y |
| LNC | 1.560 | 0.019 | 2.912 | −0.061 | Y |
| Deciduousness | 0.012 | −0.004 | 1.000 | 0.000 | N |
| Compoundness | 0.135 | 0.052 | 0.961 | −0.030 | Y |
| Nitrogen fixation | 0.000 | 0.000 | 0.612 | −0.110 | Y |
Intercepts and slopes are shown. Significant parameters are shown in bold. Traits are considered to have a funnel shape (Y = yes, N = no) if both slopes have a significant but opposite sign or if one of the slopes is significantly positive or negative, and the other slope does not statistically differ from 0. Please note that LS has a diverging funnel shape, whereas the other traits have a converging funnel.
Fig. 1.
Recovery of CWM functional trait values with time since abandonment. CWM trait values were calculated by weighting by basal area. (A) WD, (B) SLA, (C) LS, (D) LNC, (E) deciduousness, (F) compoundness, and (G) proportion NF trees. Each line represents the model prediction for a different chronosequence. Other predictors were kept constant at the mean. Lines and dots are color-coded according to the forest type as dry deciduous forest (orange) and wet evergreen forest (blue). Dots indicate individual plots. (Insets) Model predictions of an “average” dry deciduous forest CWA = −700 mm/y) and an “average” wet evergreen forest (CWA = −250 mm/y) based on the fixed effects only. Letters in Inset charts indicate whether forest age since abandonment (A), CWA (W), and their interaction (A*W) are significant.
Linear mixed models showed that all CWM trait values but SLA and LNC were significantly affected by CWA (Fig. 2); wetter sites had significantly larger LS, a lower WD, and a lower proportion of deciduous, compound, and NF trees than drier sites (Figs. 1 and 2).
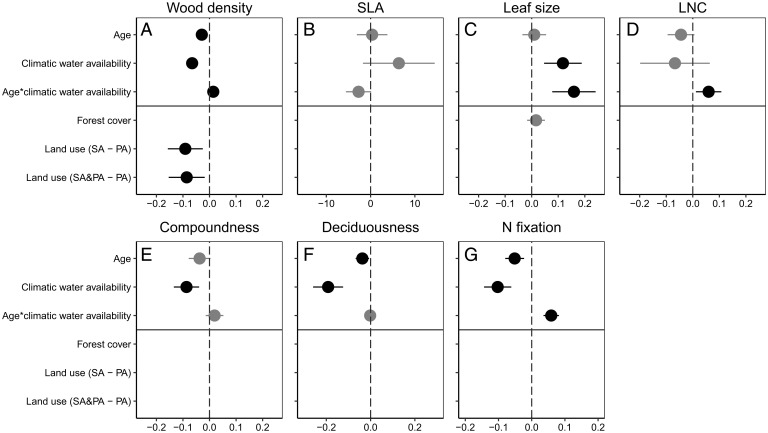
Fig. 2.
Effects of a core model (forest age, CWA, the interaction between CWA and forest age) and the potential effects of forest cover, previous land use type, CEC, and their interactions with forest age on CWM trait recovery in Neotropical secondary forests. (A) WD, (B) SLA, (C) LS, (D) LNC, (E) compoundness, (F) deciduousness, and (G) proportion NF trees (N fixation). Forest cover, previous land use type, and CEC are only included and shown when they are part of the best model. Standardized coefficients with 95% CIs are shown. Note that predictor variables were standardized but the response variables not, which explains why, for example, for SLA effect, sizes are larger. Black symbols indicate significant responses, and gray symbols indicate nonsignificant responses. Negative coefficients indicate a negative effect, and positive coefficients indicate a positive effect. Effect sizes of land use type comparisons are not directly comparable with those of the other predictors because they are dummy variables. We used PA as a reference. SC, shifting cultivation; SC and PA, some plots shifting cultivation and some plots PA. CEC was not included in any of the best models and therefore not shown.
Model prediction lines showed that the direction and slope of successional change in CWM trait values varied strongly across sites, showing both positive, flat, and negative relationships (Fig. 1). The linear mixed model indicated that for four traits (NF, WD, LS, and LNC), successional changes were predicted by CWA; there was a significant interaction between age and climatic water availability (Fig. 2). These interactions are visualized as model prediction lines for dry (orange line) and wet (blue line) forests in Fig. 1, Insets. Dry and wet forests showed opposite successional patterns for the proportion of NF trees, whereas dry forests showed a decrease over time and wet forests an increase (Fig. 1G and Table 2). Dry and wet forests showed contrasting successional patterns for WD, LS, and LNC in which one forest type showed successional change and the other not. WD and LS increased over time in wet forest but remained constant in dry forest (Fig. 1 A and C), whereas LNC decreased over time in dry forest but remained constant in wet forest (Fig. 1D). There was no significant interaction between age and climatic water availability for SLA, although for some individual dry forests, the SLA significantly increased over time, and for some wet forests, SLA significantly decreased over time (Fig. 1B and Table 2). As a result, the values of all these 5 traits converged over time, and dry and wet forests became more similar in their functional characteristics (Fig. 1 and Table 1). Dry and wet forests showed similar successional patterns for the proportion of deciduous trees and compound-leaved trees, that decreased over time, independent of CWA (Figs. 1 E and G and 2).
Table 2.
Successional responses in basal area–weighted CWM trait properties
| Dry | Wet | All | ||||||||||
| Trait | − | + | Sign | N | − | + | Sign | N | − | + | Sign | N |
| (%) | (%) | (%) | (#) | (%) | (%) | (%) | (#) | (%) | (%) | (%) | (#) | |
| WD | 18 | 9 | 27 | 11 | 0 | 21 | 21 | 19 | 7 | 17 | 23 | 30 |
| Deciduousness | 27 | 9 | 36 | 11 | 22 | 0 | 22 | 18 | 24 | 3 | 28 | 29 |
| Compoundness | 36 | 9 | 45 | 11 | 6 | 11 | 17 | 18 | 17 | 10 | 28 | 29 |
| LS | 25 | 13 | 38 | 8 | 0 | 25 | 25 | 8 | 13 | 19 | 31 | 16 |
| SLA | 18 | 36 | 55 | 11 | 26 | 11 | 37 | 19 | 23 | 20 | 43 | 30 |
| LNC | 29 | 0 | 29 | 7 | 16 | 5 | 21 | 19 | 19 | 4 | 23 | 26 |
| Percent Fabaceae | 45 | 0 | 45 | 11 | 5 | 21 | 26 | 19 | 20 | 13 | 33 | 30 |
| All traits combined | 40 | 24 | 30 | |||||||||
For two forest types and seven functional traits, it is shown for how many chronosequences the regression slope of CWM trait values against ln(time) is significantly negative (−), positive (+), or significant (sign) (independent of the direction). The frequency of significance is presented as a percentage of the number of chronosequences evaluated. Dry forests are here defined as a forest with a dry deciduous canopy and wet forest as forests with an evergreen canopy. Also, the values for dry and wet forests combined (all) are shown. The number of chronosequences evaluated can vary with the trait considered. Bolded cells indicate for each trait the most common successional pathway in each forest type.
CEC did not significantly affect any of the CWM trait values or successional changes therein (Fig. 2). Sites with a higher surrounding forest cover tended to have larger leaves (Fig. 2C) and a narrower WD range (Fig. 3A), although this was not significant. Previous land use had only a significant effect on wood density; forests regenerating on abandoned agricultural fields had lower wood densities than pastures but a stronger successional increase in WD over time (Fig. 2).
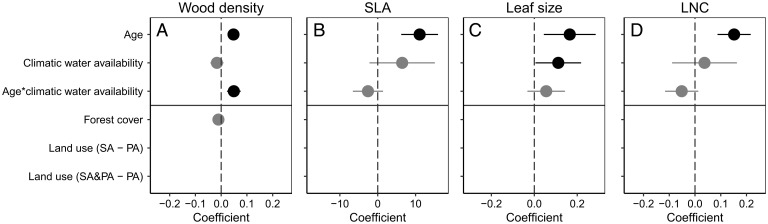
Fig. 3.
Effects of a core model (stand age, CWA, and the interaction between CWA and stand age) and the potential effects of forest cover, previous land use and CEC on recovery of within-plot trait range in Neotropical secondary forests. (A) WD, (B) SLA, (C) LS, and (D) LNC. The range is calculated per plot as the trait value of the 90th percentile minus the trait value of the 10th percentile of basal area–weighted trait values in a plot. For further explanations, refer to the legend of Fig. 2.
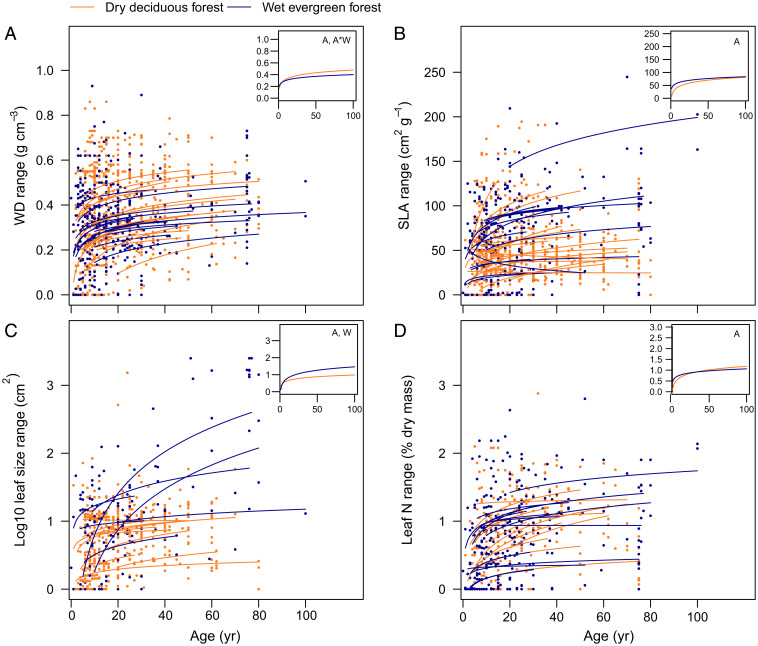
For all four continuous traits (WD, SLA, LS, and leaf nitrogen), the range in trait values per plot increased over time (Figs. 3 and 4). Only for WD range was there a significant interaction between age and climatic water availability, indicating that the WD range per plot increases more rapidly over time for wet forests than for dry forests (Fig. 4).
Fig. 4.
Range of trait values observed within communities versus time since abandonment. (A) WD, (B) SLA, (C) LS, and (D) LNC. The range is calculated per plot as the trait value of the 90th percentile minus the trait value of the 10th percentile of basal area–weighted trait values in a plot. Each line represents the model prediction for a different chronosequence. Lines and dots are color-coded according to the forest type as dry deciduous forest (orange) and wet evergreen forest (blue). Dots indicate individual plots. (Insets) The model prediction line for an “average” dry deciduous forest (CWA = −700 mm/y) and an “average” wet evergreen forest (CWA = −250 mm/y). Letters in Inset charts indicate whether forest age since abandonment (A), CWA (W), and their interaction (A*W) are significant.
When CWM trait values were calculated by weighing by the abundance of the species, more frequently significant site relationships with time were found (40% of the cases, SI Appendix, Table S3) compared to weighing by basal area (30% of the cases, Table 2).
Discussion
Community Traits Are Strongly Linked to Macroclimate.
At the start and during most part of succession, community mean trait values varied widely across sites, and this variation was associated with climatic water availability, whereas soil fertility was not selected as an explanatory variable in our model, although other not measured soil variables may be important (Fig. 2). Dry forests are characterized by harsh, hot, and dry conditions with low soil water potentials and high vapor deficits, and these conditions are even further exacerbated during the dry season and in open fields early in succession (16). Dry forest trees use a suite of strategies to avoid, delay, or tolerate drought. First, dry forest trees may avoid dry-season drought stress by having a deciduous leaf habit (Fig. 1E), and some species may store water in soft-wooded stems (24). Although average CWM WD tends to be rather high early in dry forest succession, the range in WD is also large, indicating that soft-wooded species are present (Fig. 4A). Second, dry forest trees may delay drought stress by reducing heat load and transpirational water loss by having 1) compound (bi)pinnate leaves (Fig. 1F) with heliotropic leaflets that may close at high irradiance, 2) small leaves and leaflets (Fig. 1C) that have a thin boundary layer which facilitates convective heat loss and reduces the need for transpirational cooling (25), 3) low SLA leaves (although nonsignificant in our study, Fig. 1B) that have a low surface to volume ratio that reduces evaporational water loss, and 4) a somewhat higher LNC (Fig. 1D) and, hence, Rubisco concentration, which draws down the internal CO2 concentration, creates a steeper CO2 diffusion gradient with the outer atmosphere and leads to higher CO2 influx, and requires therefore a lower stomatal aperture, resulting in less transpirational water loss (26). Third, dry forest trees may physiologically tolerate drought stress by having a high stemwood density (Fig. 1A), which is associated with narrow vessels and narrow pit pores, that are less vulnerable to drought-induced cavitation, impairment of water transport, and desiccation (27, 28). NF species dominate in dry forests, especially early in succession (Fig. 1G, cf. ref. 29), probably because nitrogen fixation allows them to have high LNCs and a deciduous leaf habit, as they can easily replace nitrogen losses from shed leaves (29). Nitrogen fixation and leaf replacement are energetically costly processes that are more easily done in high light environments (30). Perhaps this need for high light conditions explains why NF species (of which nearly all belong to the Fabaceae) are so successful in dry forests (Fig. 1G) that have a relative open canopy and more light in the lower forest strata and especially early in succession when light availability is still high (16).
In sum, dry and wet forests have a different suite of trait values, whereas dry forests have a trait value that increase drought avoidance (deciduousness), drought delay (high NF and LNC, compound leaves, small LS, and low SLA) or drought tolerance (high WD), which is important in dry environments. These trait values come, however, at the expense of fast growth, which is especially important in wet environments, and wet forests have therefore more acquisitive trait values.
Successional Changes in Community Traits.
We hypothesized that wet and dry forests would show different successional pathways in functional composition because they differ in the traits of the regional species pool and in (a)biotic conditions at the start of succession, whereas those conditions become more similar over time. Different successional pathways (i.e., a significant interaction between age and CWA) were indeed found for four out of seven traits; NF, LNC, WD, and LS (Figs. 1 and 2).
NF.
Opposite successional patterns were found for nitrogen fixers. In dry forests, the proportion of NF (Fabaceae) trees decreased over time, probably because these drought- and heat-adapted species are replaced by more shade-tolerant species when microclimatic conditions become more benign (29). In wet forests, the proportion of NF trees slightly increased over time, for which we do not have a clear explanation. The ability to fix nitrogen should especially be advantageous in early successional, nutrient-poor soils in which repeated burning and cultivation has led to increased volatilization and nutrient leaching (30). Hence, we expected in wet forest the proportion of N fixers to be higher early in succession. Because symbiotic nitrogen fixation is an energetically costly process, we also had expected that nitrogen fixers would lose their competitive advantage over time, especially in wet forests that become rapidly shaded and would therefore decrease in relative abundance. NF trees are on the other hand very plastic, and they can down-regulate their nitrogen fixation rates in shaded conditions when carbon becomes a limiting resource (30).
Contrasting successional patterns were found for LNC, WD, and LS in which one forest type showed successional changes and the other not.
LNC.
In dry forests, successional patterns in LNC paralleled the successional patterns in nitrogen fixation, as both declined over time (Fig. 1 D and F). LNC can be high early in succession because nitrogen fixers comprise over half of the basal area. Nitrogen fixers have high LNCs that increase photosynthetic water use efficiency, which should especially be advantageous in (early successional) dry environments (26).
WD.
Community WD increased over time in wet forests in line with standard successional theory (Fig. 1A, cf. ref. 17), whereas it remained constant in dry forests (Fig. 1A). In contrast, Poorter et al. (17) found for a larger set of 50 sites, including the current 30 sites, that WD decreased over time in dry forests. This is probably because early in dry forest succession, harsh climatic conditions and strong edaphic and atmospheric drought select for dense-wooded species with high cavitation resistance, whereas later in succession, more benign, cool, and humid understory conditions allow for the establishment of species with lower WD and low cavitation resistance.
LS.
LS increased over time in wet forests. In hot, early successional environments, small-sized leaves have as advantage that they have a small boundary layer, which facilitates latent leaf loss and avoids overheating (25). During succession, the vegetation builds up, which leads to improved microclimatic conditions and a release on the constraints of LS, while increased shading and light competition may select for species with larger leaves that are more efficient in light capture. We did not find such a successional pattern in dry forest, probably because at the onset of succession, >90% of the trees avoid dry-season heat by being deciduous (Fig. 1E), and 80% of the trees have compound leaves (Fig. 1F) that already reduce heat load by having tiny leaflets.
SLA.
In general, there was no significant age × CWD interaction effect on SLA (Fig. 2), although several individual wet and dry sites did show contrasting patterns. In some of the wet forests included in this study, SLA significantly decreased over time (Fig. 1B and Table 2) (15) in line with successional theory. Early successional acquisitive pioneers have inexpensive, short-lived leaves with a high SLA (31) that increase short-term light capture and growth potential in a high light environment. Over time, they are replaced by late successional conservative shade-tolerant species with dense, thick, and well-protected leaves that enhance leaf longevity, carbon gain, and plant survival in shaded environments (32). In contrast, in some of the driest forests, SLA significantly increased over time, probably because during succession, extremely drought-adapted species are replaced by more competitive species when environmental conditions become more benign (Table 2).
Deciduousness and compoundness.
In both dry and wet forests, two traits showed similar successional pathways (i.e., there was no significant interaction between age and CWA, Fig. 2). The proportion of deciduous and compound-leaved trees decreased over time (Fig. 1 E and G), probably because deciduous and compound-leaved species are better adapted to drought and heat. In both forest types, they are replaced by more shade-tolerant species (with evergreen and simple leaves) when microclimatic conditions become more benign. An evergreen leaf habit is advantageous in benign environments, as it allows for a longer growing season, while simple leaves allow for the investment in photosynthetically active leaf lamina rather in costly rachae (33).
In sum, dry and wet forests show different successional pathways for a number of traits. In dry forests, species turnover seems to be driven by drought tolerance traits, which are especially important early in succession (i.e., high NF, LNC, and low SLA), and in wet forests, species turnover is driven by shade tolerance traits, which are especially important later in succession (i.e., high WD, large leaves, and low SLA).
Drivers of Community Assembly.
Community assembly is driven by a hierarchy of drivers that operate from regional to local scales. Given the nature of our study (a broad-scale geographic comparison) and the nature of our data (environmental conditions within sites were quantified less precisely), the regional drivers turned out to be more important. At the regional scale, climatic water availability clearly had a strong effect on community functional composition, as it sets an upper boundary to the length of the growing season and determines canopy phenology (dry deciduous versus evergreen forests) and seasonal dynamics in population processes (recruitment, growth, and survival) and ecosystem processes (34). Across these Neotropical forests, soil fertility (CEC) had no additional effect, probably because it is less fundamental for plant survival compared to water availability or perhaps because it shows substantial heterogeneity at smaller spatial scales that we did not measure. Other studies do show that small scale soil heterogeneity affects community functional composition (35). At the landscape scale, forest cover in the surrounding landscape determines the availability of seed sources and dispersal agents and improves the microclimate. Forest cover was only included in two trait models, although it was not significant; it tended to increase LS and tended to reduce the WD range (Figs. 2 and 3). High forest cover landscapes may have more animal-dispersed old growth species with larger leaves that can recruit in secondary forests, or these landscapes may reduce heat load and increase humidity, which facilitates the success of large-leaved species. A reduced WD range may reflect a lower abundance of extremely soft-wooded pioneer species in these intact forest cover landscapes (36). At a local scale, previous land use intensity and its associated disturbances may affect the species that can regenerate. Pastures had a higher WD than agricultural fields (Fig. 2A), which can be the result of remnant old growth trees that were left to provide shade for cattle or the strong filter imposed by annual burning of pastures, which only allows resprouting trees to regenerate (37), that tend to have a high WD. Local studies do show that landscape context and land use intensity have a strong effect on community assembly (20, 38). A standardized and detailed quantification of the landscape context and land use intensity is therefore a priority to improve future large-scale comparative studies (8).
Convergence in Trait Values over Time?
Across all plots, community trait values of most traits showed a funnel-shaped relationship with age, with large trait variation early in succession and less trait variation later in succession leading to a “convergence” of trait values with age (Fig. 1 and Table 1). This funnel shape is the result of counteracting community assembly processes that operate at three different spatial scales; at the plot scale (i.e., a patch), at the site scale (i.e., a chronosequence), and among sites. At the plot scale within sites, trait variation actually increases with age (Figs. 3 and 4), probably because of strong dispersal limitation and environmental filtering early in succession versus increased competitive interactions and limiting similarity later in succession (23) or because more species with extreme trait values arrived at the site. At the site scale, visual inspection of the data suggests that trait variation across plots within such a landscape tends to decrease over time. This is probably because early in succession, a combination of dispersal limitation, few species, and strong species dominance lead to large variation in initial community composition from place to place, as the few founding species may differ largely in their trait values. Later in succession, the plots converge in their trait values, as most species have been able to disperse to the different plots and because changed environmental conditions select for species with more conservative or acquisitive trait values depending on CWA. Among sites, trait variation decreases with age time, probably because strong environmental differences (i.e., drought) lead to striking differences in the start of succession (Fig. 1), whereas over time, these environmental differences become reduced when forest cover builds up, and the understory microclimate becomes more benign. As a result, dry and wet forests differ in their pathway of succession (Fig. 1), leading to a convergence in community trait values over time. Funnel-shaped relationships with age could also arise from a sampling effect, when an increase in species number may lead to a more central CWM trait value, as the traits are averaged across many species. We tested for this idea for WD, but the species sampling effect did not play a role (39).
In sum, community assembly processes at the plot level may lead to modest trait divergence, whereas community assembly processes at the landscape scale and continental scale may lead to stronger trait convergence, leading to the funnel-shape relationship of traits with time (Fig. 1 and Table 1).
Little Successional Changes for Many Traits and Chronosequences.
A functional trait approach has the potential and appeal to infer patterns and mechanisms in community assembly (40) because traits are closely linked to species performance in a given environment. We studied secondary tropical forest succession after agricultural abandonment, which arguably presents one of the most ideal systems to study community assembly because nearly all vegetation is removed, and community assembly starts from nearly scratch, although there are of course legacies in the soil and the propagule bank. Additionally, community assembly proceeds rapidly in tropical forests, as, in general, there is a rapid, predictable buildup of vegetation with concomitant changes in environmental conditions and species composition if soils are not degraded and if sufficient seed sources are nearby. Although several traits showed clear successional patterns (see section Successional Changes in Community Traits), out of the 190 evaluated trait–chronosequence relationships, between 60 (for abundance weighting, SI Appendix, Table S2) and 70% (for basal area weighting, Table 1) of the evaluated trait–chronosequence relationships were not significant, and successional patterns were less straightforward than we expected them to be. Several methodological and ecological reasons may explain this surprising lack of significant results. First, to infer successional patterns, we used a chronosequence approach, which uses a space-for-time substitution. This approach has as advantage that long time periods can be considered, but it assumes that all plots started under similar conditions. This is not necessarily the case, as initial species composition may vary strongly from place to place because of the initial founder (i.e., priority) effects of species that can regenerate locally very abundantly. A longitudinal approach in which plots are monitored over time may therefore reveal stronger successional trait patterns. Second, we used a species-based approach to assign functional trait values to a certain stand rather than measuring traits of individual trees. As traits respond plastically to environmental conditions, successional patterns in trait values could have been more clear if we would have measured trait values for each individual tree in a stand, which may improve the ability to detect community assembly mechanisms (22, 41), although measuring traits of each individual tree is logistically challenging. Third, several plots contain old growth species that were present prior to agriculture use and have resprouted. As these old growth species have different trait values than pioneers that regenerate from seed, this may blur successional trends in community trait values, especially so in dry forests where resprouting is a common mode of regeneration. Fourth, opposite successional pathways for some traits in dry and wet forest (e.g., a significant increase in trait values in dry forest and a decrease in wet forest) means per definition that at intermediate rainfall conditions, there are little successional changes. Fifth, leaf traits show less frequently significant successional change than expected, ranging from 23 to 43% of the evaluated cases (Table 1). This is surprising, given that SLA and LNC are thought to play a pivotal role in global plant strategies (42) and shade tolerance (32) and given the widespread belief that leaf traits should drive succession, as they are important for light capture and carbon gain in dense stands with competing plants. Perhaps leaf traits show little successional change because 1) SLA is important for seedlings but less important for the carbon gain and growth of large trees (43), which weight strongly in our CWM calculations; 2) the role of traits in light competition may be more pronounced at the tree level at which competition occurs rather than at the stand level that we evaluated here; 3) we did not account for light-dependent acclimation, which can especially be strong for SLA; 4) organ-level traits such as leaf traits may respond less to succession than integrative traits that reflect the strategy of the whole individual and that can be the result of various combinations of traits (44); and 5) succession may be more driven by other factors than light, such as soil fertility, drought, pests, pathogens, dispersal limitation, or chance (45).
Trait Ranges.
We hypothesized that harsh environmental conditions early in succession filter for communities with a narrow range of trait values, whereas benign conditions later in succession allow for a wider range of possible trait values. Additionally, the accumulation of new species over time (46) may also increase the likelihood to include a larger diversity and range of trait values. We indeed found that, for most sites, within-community variation in individual traits increased with age (Fig. 4) and that forest age had a significant positive effect on trait ranges (Fig. 3). In sum, increased trait variation with forest age may be caused by 1) less stringent environmental filtering, 2) larger diversity over time because of competitive interactions and limiting similarity (23), 3) a wider range of niche opportunities in a structurally more complex vegetation (47), and 4) species accumulation over time (because of dispersal limitation) with some species having different trait values. We hypothesized that wetter forests would have a wider trait range than drier forests because they have more benign macroclimatic conditions, a taller canopy, a steeper light gradient, and more species, which together allow for a greater leaf trait diversity and more extreme trait values. This was only confirmed for LS (Fig. 3C). WD range increased more strongly with age for wetter forests (Fig. 3A), probably dense-wooded, shade-tolerant species established below the canopy of soft-wooded pioneer species, thus expanding the WD range.
Consequences for Ecosystem Functioning.
During succession, forests can recover rapidly in biomass (48) and ecosystem functioning, with stocks and fluxes of carbon and nutrients increasing when biomass builds up (49). Although biomass is the strongest driver, successional shifts in trait values may also have large impacts on ecosystem functioning. In dry forests, the proportion of nitrogen fixers, deciduous and compound species, and the LNC decrease with forest age (Fig. 1). This may slow down carbon and nutrient cycling because of slower symbiotic N fixation and slower decomposition of tough, evergreen, nutrient-poor litter (50). In wet forests, an increase in WD and LS increase with forest age, and deciduous and compound species decrease with forest age, which may slow down biogeochemical cycling, as high WD stems are longer lived and more difficult to decompose; it may also increase carbon stocks. In both forests, the negative effect of community trait changes may therefore partly offset the positive effects of increased aboveground biomass on biogeochemical cycling. Increased trait variation during succession in both dry and wet forests (Fig. 4) may reflect an increase in niche complementarity, which may lead to more efficient resource use and higher community-level productivity (51). Increased trait variation may also buffer ecosystem functioning to environmental change and enhance ecosystem resilience (52).
Implications for Restoration.
Forest restoration efforts should ideally rely on natural regeneration, as this is economically and ecologically most efficient, with the highest gains for biodiversity, ecosystem functioning, and ecosystem services (4, 53). When unassisted natural regeneration is not possible because of a lack of seed propagules, a deteriorated microclimate, or degraded soils condition, then restoration efforts should rely on assisted natural regeneration or on active restoration based on tree plantings. Tree plantings that use mixes of pioneer and late successional species have been successful in kick starting succession and creating an environment that favors natural regeneration of later successional species (54). The species selected for these mixes should meet multiple social, economic, and ecological criteria but at least be able to survive and thrive under the local environmental conditions, as our results indicate that the start and pathway of secondary succession vary strongly with macroclimatic conditions. Based on our results, we propose a science-based framework for species selection for restoration in which functional traits can be used as simple and straightforward proxies to select appropriate species (cf. ref. 55). First, this means that early successional species selected for the initial vegetation layer in drier forests (precipitation <1,500 mm/y) should be drought tolerant and possess (a combination of) traits such as high WD, deciduous leaf habit, compound leaves, high LNC, and low SLA. In wetter forests, species should be fast growing and possess the opposite suite of trait values as in drier forests (Figs. 1 and 2). In both drier and wetter forests, it is beneficial to plant NF species (cf. ref. 30 but refer to ref. 56) (Fig. 1G). Second, later successional species selected for the secondary layer should have contrasting values in WD, compoundness, and SLA compared to the early successional species selected for the initial layer. This means that in drier forests, these late successional species should have low WD, high SLA, and often simple leaves, whereas in wetter forest it is the other way around (Fig. 1 A, B, and F). Third, young secondary forests display a large range in trait values. For example, in 20-y-old forest plots, coexisting species show an average range of 0.3 g ⋅ cm−3 in WD, 50 cm2 ⋅ g−1 in SLA, 0.7% in leaf nitrogen, and an order of magnitude in LS (Fig. 4). This natural trait range can be used as an indicator to select a mix of species that differ sufficiently in their trait values.
Conclusions.
We have advanced forest successional theory by synthesizing continental-scale patterns in succession using a functional trait approach. Dry and wet forest communities have opposite suites of traits reflecting a trade-off between 1) conservative trait values that increase drought avoidance, delay, and tolerance in seasonally dry environments and 2) acquisitive trait values that increase growth rates in wet environments. Dry and wet forests show different successional pathways for a number of traits; in dry forests, succession is driven by drought tolerance traits, whereas in wet forests, it is driven by shade tolerance traits. These results have large implications for our understanding of succession and for active forest restoration strategies.
Materials and Methods
The methods are partly derived and modified from Rozendaal et al. (46) and Poorter et al. (17).
Study Sites.
We compiled chronosequence data for 30 Neotropical lowland forest sites (48) covering the entire latitudinal gradient in the Neotropics (SI Appendix, Fig. S1 and Table S1). We focused on the Neotropics, that is, tropical South America and Mesoamerica, because 1) shifting cultivation is an important land use type there, 2) the region has a relatively shared biogeographic history, thus reducing confounding historical effects, and 3) many chronosequence studies have been established in the area. Annual rainfall varied from 750 to 4,000 mm ⋅ y−1 across sites, topsoil CEC from 4.9 to 64.6 cmol(+) ⋅ kg−1, and percent forest cover in the landscape matrix ranged from 23 to 100% (SI Appendix, Table S1).
Plots.
On average, 34 plots (range 5 to 274) were established per chronosequence, with the age of the youngest plot ranging from 0 to 20 y in time since abandonment. The age range covered by chronosequence plots varied from 12 to 80 y across sites (SI Appendix, Table S1), and plot sizes ranged from 0.01 to 1 ha, with an average of 0.1 ha across all plots. Per site, plots were of the same size. For trees, palms, and shrubs, all stems ≥5 cm stem diameter at breast height (dbh) were measured for dbh and identified to species, except for six sites in which minimum dbh was 10 cm. Across chronosequences, on average, 94.5% of stems were identified to species (range 71 to 100%) and 99.5% (range 94 to 100%) to family, genus, or morphospecies.
Traits.
We focused on seven key traits that are important both as response traits (indicating how communities are assembled during succession, SI Appendix, Table S2) and as effect traits (determining how ecosystems function in terms of carbon, water, and nutrient stocks and cycling).
SLA (in centimeters2 ⋅ gram−1) is the leaf area per unit leaf dry mass. It indicates the biomass efficiency of leaf display for light capture. Higher SLA species tend to have shorter leaf lifespan. SLA reflects therefore a trade-off between efficient leaf area deployment versus a long duration of the photosynthetic revenues from the leaf. LNC (in %) is the nitrogen mass per unit leaf dry mass and is inversely related to leaf lifespan. It reflects therefore a trade-off between species with low nitrogen concentration that conserve nutrients, and species with high nitrogen concentrations have high photosynthetic capacity and carbon gain. LS (in centimeters2) is the vertically projected lamina of the leaf. It reflects a trade-off between the ability to dissipate heat and, hence, reduce transpiration in small-leaved species versus efficient light capture and outshading of competing neighbors for large-leaved species. Leaf compoundness indicates whether leaves consist of several leaflets (pinnate, bipinnate, or palmately compound). It reflects a trade-off between cheaper construction costs for simple leaves versus the ability to dissipate heat, close leaflets, have more gradual leaflet abscission, and reduce water loss for compound-leaved species. Leaf deciduousness indicates whether species have a partially or completely leafless crown for at least several weeks in the dry season. It indicates a trade-off between avoidance of dry-season drought stress in deciduous species versus a longer, year-round growing season for evergreen species. WD (in g ⋅ cm−3) is the wood dry mass divided by the wood green volume. It reflects a trade-off between fast volumetric growth of soft-wooded species and high survival because of resistance against biophysical hazards and drought in dense-wooded species. We also evaluated whether a tree species has the potential to fix atmospheric nitrogen using the literature, as N is often a limiting factor in degraded soils of abandoned agricultural fields. This does not necessarily mean that nitrogen fixation occurs at substantial rates, as nitrogen fixation is an energetically costly process, and it can be down-regulated under shaded conditions (30). Many of these traits are (loosely) associated in plant syndromes or plant strategies in which high values of SLA, LNC, LS, and simple evergreen leaves and soft wood are associated with high resource acquisition and fast growth and returns on investment, whereas the opposite trait values are associated with resource conservation and persistence (19).
Successional patterns in WD and nitrogen fixers have been analyzed before for a larger number of sites (17, 29), but they have been included here to provide a completer overview of trait responses to evaluate whether successional patterns in leaf traits mirror those of a stem trait and to check whether the same successional patterns reported before hold for our subset of 30 sites for which most of the seven traits were available.
Species-specific trait data were collected for 28 sites (SI Appendix, Table S1) (57) and for two sites from nearby areas. Trait measurements could differ between sites, as traits were initially collected with different objectives. Some sites collected leaf trait data (i.e., LS and SLA) based on the whole leaf, whereas others did so for the smallest functional leaf unit (the whole leaf for simple-leaved species but the leaflet in case of compound-leaved species). To be able to compare the SLA of different sites, we used for the latter sites a regression equation to convert the SLA of leaflets of compound-leaved species to whole-leaf SLA based on three datasets for which SLA was measured in both ways; SLA_leaf = 67.76755 + 0.40535 × SLA of the functional unit (adjusted R2 = 0.59, P < 0.0001). Some sites included the petiole in the SLA calculations, whereas others excluded them. For LS comparisons, we only included those sites that had measured the size of whole leaves. Different methods may lead to differences in the intercepts between studies, but they have little effect on the successional pattern detected within a site.
Community Functional Composition.
For each plot, we calculated community functional composition based on species-specific trait values. Traits can be plastic and respond to environmental gradients. To take trait acclimation and adaptation to local site conditions into account, trait data were locally collected at each site with the exception of Salvatierra and San Lorenzo for which traits from nearby sites were used. Because trait data were collected at the site level and not at the plot level, plasticity in response to successional stage could not be accounted for. In general, within-species plasticity in WD is small (coefficient of variation of 5 to 9%), whereas the plasticity for LNC (8 to 19%) and SLA (8 to 16%) are larger, and for LS, it is largest (17 to 36%) (58). Successional changes in community trait values as reported here are therefore only due to species turnover and not due to plasticity.
Species-specific WD data were collected for 30 sites. When some local species data were not available, we used the average local site data at genus or family level, as WD values of tropical trees are strongly phylogenetically conserved (59).
For each plot, we calculated CWM trait values in two ways: based on species-level trait values weighed by 1) the proportional basal area or 2) the proportional abundance of the species in the plot. Weighing by basal area reflects the dominance and hence, ecological success of species during community assembly. It also informs about ecosystem functioning because basal area scales closely with total leaf area and with water transport capacity of trees and therefore with the effects that trees have on ecosystem functioning. Weighing by abundance provides additional information on community assembly, as stems of small understory species and tall canopy species weigh equally in the analysis, thus excluding the effect of stem size and dominance. Because of its log-normal distribution, species-specific LS was log10-transformed, after which the CWM was calculated. To describe trait variation in each community, we calculated for each plot trait ranges based on 100 trait values for which each single trait value corresponds to the species trait value associated with 1% of the plot basal area. The trait range was calculated as the difference between the 10th and the 90th percentiles of these 100 values. For stem density, we used the same approach. For a description of annual rainfall (millimeters ⋅ year−1), CWA (in millimeters ⋅ year−1, also referred to as climatic water deficit), and CEC (in centimoles(+) ⋅ kilogram−1), refer to SI Appendix, SI Methods Environmental Conditions.
Statistical Analyses.
Successional changes in functional composition were assessed for each chronosequence using secondary forest plots only. To assess site-specific changes in functional composition, we related for each trait the functional properties of the plot (CWM traits) to forest age since abandonment using linear regressions (Table 2). Age since abandonment was ln-transformed prior to analysis because forest structure, environmental conditions, and species composition typically change nonlinearly over time with rapid initial changes and slow changes afterward. The regression slope indicates direction and pace of functional change during succession. We used the site-specific regression equations to predict CWM trait values at 5 y, reflecting the early successional community that is filtered out by the macroenvironment.
To assess how different factors affect CWM trait values and trait ranges, we use linear mixed-effects models. Because there are many predictor variables and a relatively limited dataset of 30 sites, we started with a core model to test our main research question of whether successional pathways of community functional properties differ between wet and dry forests. The core model included forest age, CWA, and their interaction as fixed effects and a random intercept and slope for ln(age) per site. We compared this core model with a range of other models that additionally included various combinations of soil CEC (an indicator of soil fertility), surrounding forest cover, previous land use, the interaction between forest age and forest cover, and the interaction between forest age and previous land use. Previous land use consisted of pasture (PA), shifting agriculture (SA), or a combination of these (SA+PA). PA was used as a reference value. Continuous predictors were standardized by subtracting the mean and dividing by the SD to be able to compare effect sizes. Models were compared using Akaike’s information criterion adjusted for small sample sizes (AICc). From the best models, thus the model with the lowest AICc and models within two AICc units from that model, which we regarded as equally supported, we selected the most parsimonious model (i.e., the model with the lowest number of predictor variables). To visualize how dry and wet forests differ in successional changes in community functional properties, we used the obtained model equations to make prediction lines of trait values against forest age for an “average” wet forest (CWA = −250 mm/yr) and an “average” dry forest (CWA = −700 mm/yr). These reference values of CWA are close to the average values, and the midpoint of CWA range for our 15 seasonally dry forests and 15 evergreen wet forests. (SI Appendix, Table S1). To test whether, across all sites, community trait values show funnel-shaped relationships with time, we did for each trait a quantile regression to estimate the slope for the 10th and 90th percentile (Table 1). Traits do show a funnel shape if both slopes have a significant but opposite sign or if one of the slopes is significantly positive or negative, and the other slope does not statistically differ from 0. All analyses were performed in R 3.3.2. Community-weighted mean trait values were calculated using the Functional Diversity package (60).
Supplementary Material
Acknowledgments
This paper is a product of the 2ndFOR collaborative research network on secondary forests (https://sites.google.com/view/2ndfor/home) and paper number six from 2ndFOR. We thank the owners of the secondary forest sites and the local communities for access to their forests, all the people who have established and measured the plots, and the institutions and funding agencies that supported them. We thank the following agencies for financial support: Netherlands Foundation for Scientific Research (NWO-ALW.OP241), European Research Council Advanced Grant PANTROP 834775 and Macquarie University Visiting Researcher Grant to L.P., Wageningen University and Research (International Research and Education Fund, FOREFRONT), Netherlands Foundation for Scientific Research (NWO-ALW.OP457), NWO-FAPESP (São Paulo Research Foundation) Grant 17418 (NEWFOR), the National Science and Engineering Research Council of Canada, Inter-American Institute for Global Change Research Collaborative Network Program CNR3-025, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq - PQ 307422/2012-7, PQ 309965/2016-0) Consejo Nacional de Ciencia y Tecnología (CONACYT) (CB-2009-01-128136), Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica-Universidad Nacional Autónoma de México (IN216007, IN218416, IN207618, IN201020, IN217620), Fondo Mixto Consejo Nacional de Ciencia y Tecnología-Gobierno del Estado de Yucatán (Yuc-2008-C06-108863), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (PPM-726-16), DAAD (German Academic Exchange Service), Rio Grande do Sul Research Foundation (No. 2218 – 2551/12-2), FAPESP (Grant No. 2014/14503-7), the National Council for Scientific and Technological Development of Brazil (Grant No. 304817/2015-5 and 309874/2015-7), and the Coordination for the Improvement of Higher Education Personnel of Brazil (Grant No. 88881.064976/2014-01) to P.B. We thank the reviewers for their constructive comments that have improved the manuscript.
Footnotes
Author contributions: L.P., D.M.A.R., M.T.v.d.S., and M.W. designed research; L.P., D.M.A.R., F.B., d.J.S.A., F.S.A., J.L.A., L.F.A.V., J.M.B., R.B., V.B., P.H.S.B., R.G.C., J.C., R.L.C., G.D.C., D.C., B.H.J.d.J., J.S.D., D.H.D., S.J.D., E.D.G., J.M.D., S.M.D., M.M.E.S., G.W.F., B.F., V.G.M., J.S.H., J.L.H.-S., C.C.J., D.K., E.L.-T., S.G.L., M.L., O.R.L., E.M.-S., M.M.-R., J.A.M., F.M., V.d.S.M., S.C.M., R. Muñoz, R. Muscarella, Y.R.F.N., S.O.-G., H.P., A.S.-A., L.S.-V., M.T., M.U., L.P.U., M.v.B., M.T.v.d.S., M.D.M.V., S.J.W., K.J.Z., and J.K.Z. performed research; L.P., D.M.A.R., F.B., C.C.J., M.T.v.d.S., and M.W. contributed analytic tools; D.M.A.R. and M.T.v.d.S. analyzed data; L.P. wrote a first draft of the paper; and D.M.A.R., F.B., d.J.S.A., F.A., J.L.A., L.F.A.V., J.M.B., R.B., V.B., P.H.S.B., R.G.C., J.C., R.L.C., G.D., D.C., B.H.J.d.J., J.S.D., D.H.D., S.J.D., E.D.G., J.M.D., S.M.D., M.M.E.S., G.W.F., B.F., V.G.M., J.S.H., J.L.H.-S., D.K., E.L.-T., S.G.L., M.L., O.R.L., E.M.-S., M.M.-R., J.A.M., F.M., V.d.S.M., S.C.M., R. Muñoz, R. Muscarella, Y.R.F.N., S.O.-G., R.S.O., H.P., A.S.-A., L.S.-V., M.T., M.U., L.P.U., M.v.B., M.T.v.d.S., M.D.M.V., S.J.W., K.J.Z., J.K.Z., and M.W. provided feedback on preliminary analyses and commented on drafts.
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003405118/-/DCSupplemental.
Data Availability
CWM trait data and trait range data of each plot have been deposited in the Data Archiving and Networked Services repository (https://doi.org/10.17026/dans-zz5-hf3s).
References
- 1.Chazdon R. L., et al. , Carbon sequestration potential of second-growth forest regeneration in the Latin American tropics. Sci. Adv. 2, e1501639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chazdon R. L., Beyond deforestation: Restoring forests and ecosystem services on degraded lands. Science 320, 1458–1460 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Holl K. D., Restoring tropical forests from the bottom up. Science 355, 455–456 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Brancalion P. H. S., et al. , Global restoration opportunities in tropical rainforest landscapes. Sci. Adv. 5, eaav3223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements F. E., Plant Succession: An Analysis of the Development of Vegetation (Carnegie Institution of Washington, 1916). [Google Scholar]
- 6.Fukami T., Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 46, 1–23 (2015). [Google Scholar]
- 7.Garnier E., Navas M. L., Grigulis K., Plant Functional Diversity: Organism Traits, Community Structure, and Ecosystem Properties (Oxford University Press, Oxford, 2016). [Google Scholar]
- 8.Jakovac C. C., et al. , The role of land-use history in driving successional pathways and its implications for the restoration of tropical forests. Biol. Rev. Camb. Philos. Soc. 96, 1114–1134 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chazdon R. L., Second Growth: The Promise of Tropical Forest Regeneration in an Age of Deforestation (University of Chicago Press, Chicago, IL, 2014). [Google Scholar]
- 10.Bazzaz F. A., Physiological ecology of plant succession. Annu. Rev. Ecol. Syst. 10, 351–371 (1979). [Google Scholar]
- 11.Wright I. J., et al. , The worldwide leaf economics spectrum. Nature 428, 821–827 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Finegan B., Pattern and process in neotropical secondary rain forests: The first 100 years of succession. Trends Ecol. Evol. 11, 119–124 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Letcher S. G., et al. , Environmental gradients and the evolution of successional habitat specialization: A test case with 14 Neotropical forest sites. J. Ecol. 103, 1276–1290 (2015). [Google Scholar]
- 14.ter Steege H., et al. , Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 443, 444–447 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Lohbeck M., et al. , Successional changes in functional composition contrast for dry and wet tropical forest. Ecology 94, 1211–1216 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Lebrija-Trejos E., Pérez-García E. A., Meave J. A., Poorter L., Bongers F., Environmental changes during secondary succession in a tropical dry forest in Mexico. J. Trop. Ecol. 27, 477–489 (2011). [Google Scholar]
- 17.Poorter L., et al. , Wet and dry tropical forests show opposite successional pathways in wood density but converge over time. Nat. Ecol. Evol. 3, 928–934 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Baraloto C., et al. , Decoupled leaf and stem economics in rain forest trees. Ecol. Lett. 13, 1338–1347 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Reich P. B., The world‐wide ‘fast–slow’plant economics spectrum: A traits manifesto. J. Ecol. 102, 275–301 (2014). [Google Scholar]
- 20.Lohbeck M., et al. , Drivers of farmer-managed natural regeneration in the Sahel. Lessons for restoration. Sci. Rep. 10, 15038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribeiro E. M. S., et al. , Functional diversity and composition of Caatinga woody flora are negatively impacted by chronic anthropogenic disturbance. J. Ecol. 107, 2291–2302 (2019). [Google Scholar]
- 22.Bhaskar R., Dawson T. E., Balvanera P., Community assembly and functional diversity along succession post‐management. Funct. Ecol. 28, 1256–1265 (2014). [Google Scholar]
- 23.Lasky J. R., Uriarte M., Boukili V. K., Chazdon R. L., Trait-mediated assembly processes predict successional changes in community diversity of tropical forests. Proc. Natl. Acad. Sci. U.S.A. 111, 5616–5621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borchert R., Soil and stem water storage determine phenology and distribution of tropical dry forest trees. Ecology 75, 1437–1449 (1994). [Google Scholar]
- 25.Gates D. M., Transpiration and leaf temperature. Annu. Rev. Plant Physiol. 19, 211–238 (1968). [Google Scholar]
- 26.Wright I. J., Reich P. B., Westoby M., Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 15, 423–434 (2001). [Google Scholar]
- 27.Markesteijn L., Poorter L., Paz H., Sack L., Bongers F., Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 34, 137–148 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Pineda-García F., Paz H., Meinzer F. C., Drought resistance in early and late secondary successional species from a tropical dry forest: The interplay between xylem resistance to embolism, sapwood water storage and leaf shedding. Plant Cell Environ. 36, 405–418 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Gei M., et al. , Legume abundance along successional and rainfall gradients in Neotropical forests. Nat. Ecol. Evol. 2, 1104–1111 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Batterman S. A., et al. , Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 502, 224–227 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Poorter L., van de Plassche M., Willems S., Boot R. G. A., Leaf traits and herbivory rates of tropical tree species differing in successional status. Plant Biol. 6, 746–754 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Poorter L., Bongers F., Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 87, 1733–1743 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Niinemets U., Portsmuth A., Tobias M., Leaf size modifies support biomass distribution among stems, petioles and mid-ribs in temperate plants. New Phytol. 171, 91–104 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Sanchez-Azofeifa A., Powers J. S., Fernandes G. W., Quesada M., Tropical Dry Forests in the Americas: Ecology, Conservation, and Management (CRC Press, 2013). [Google Scholar]
- 35.Pinho B. X., et al. , Soil-mediated filtering organizes tree assemblages in regenerating tropical forests. J. Ecol. 106, 137–147 (2018). [Google Scholar]
- 36.ter Steege H., Welch I., Zagt R., Long-term effect of timber harvesting in the Bartica Triangle, Central Guyana. For. Ecol. Manage. 170, 127–144 (2002). [Google Scholar]
- 37.Martínez-Ramos M., et al. , Differential ecological filtering across life cycle stages drive old-field succession in a neotropical dry forest. For. Ecol. Manage. 482, 118810 (2021). [Google Scholar]
- 38.Jakovac C. C., Peña-Claros M., Kuyper T. W., Bongers F., Loss of secondary-forest resilience by land-use intensification in the Amazon. J. Ecol. 103, 67–77 (2015). [Google Scholar]
- 39.Poorter L., et al. , Wet and Dry Tropical Forests Show Opposite Successional Pathways in Wood Density but Converge Over Time (DANS, 2019). [DOI] [PubMed] [Google Scholar]
- 40.McGill B. J., Enquist B. J., Weiher E., Westoby M., Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 (2006). [DOI] [PubMed] [Google Scholar]
- 41.Boukili V. K., Chazdon R. L., Environmental filtering, local site factors and landscape context drive changes in functional trait composition during tropical forest succession. Perspect. Plant Ecol. Evol. Syst. 24, 37–47 (2017). [Google Scholar]
- 42.Díaz S., et al. , The global spectrum of plant form and function. Nature 529, 167–171 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Poorter L., et al. , Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89, 1908–1920 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Rosado B. H., de Mattos E. A., On the relative importance of CSR ecological strategies and integrative traits to explain species dominance at local scales. Funct. Ecol. 31, 1969–1974 (2017). [Google Scholar]
- 45.Craven D., Hall J. S., Berlyn G. P., Ashton M. S., Breugel M., Environmental filtering limits functional diversity during succession in a seasonally wet tropical secondary forest. J. Veg. Sci. 29, 511–520 (2018). [Google Scholar]
- 46.Rozendaal D. M. A., et al. , Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 5, eaau3114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heidrich L., et al. , Heterogeneity-diversity relationships differ between and within trophic levels in temperate forests. Nat. Ecol. Evol. 4, 1204–1212 (2020). [DOI] [PubMed] [Google Scholar]
- 48.Poorter L., et al. , Biomass resilience of Neotropical secondary forests. Nature 530, 211–214 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Lohbeck M., Poorter L., Martínez-Ramos M., Bongers F., Biomass is the main driver of changes in ecosystem process rates during tropical forest succession. Ecology 96, 1242–1252 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Cornwell W. K., et al. , Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071 (2008). [DOI] [PubMed] [Google Scholar]
- 51.van der Sande M. T., et al. , Biodiversity in species, traits and structure determines carbon stocks and uptake in tropical forests. Biotropica 49, 593–603 (2017). [Google Scholar]
- 52.Sakschewski B., et al. , Resilience of Amazon forest emerges from plant trait diversity. Nat. Clim. Chang. 6, 1032–1036 (2016). [Google Scholar]
- 53.Crouzeilles R., et al. , Ecological restoration success is higher for natural regeneration than for active restoration in tropical forests. Sci. Adv. 3, e1701345 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues R. R., Lima R. A., Gandolfi S., Nave A. G., On the restoration of high diversity forests: 30 years of experience in the Brazilian Atlantic Forest. Biol. Conserv. 142, 1242–1251 (2009). [Google Scholar]
- 55.Thomas E., et al. , The Importance of Species Selection and Seed Sourcing in Forest Restoration for Enhancing Adaptive Potential to Climate Change: Colombian Tropical Dry Forest as a Model (Secretariat of the Convention on Biological Diversity, 2017). [Google Scholar]
- 56.Taylor B. N., Chazdon R. L., Bachelot B., Menge D. N. L., Nitrogen-fixing trees inhibit growth of regenerating Costa Rican rainforests. Proc. Natl. Acad. Sci. U.S.A. 114, 8817–8822 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swenson N. G. U., et al. , Interspecific functional convergence and divergence and intraspecific negative density dependence underlie the seed-to-seedling transition in tropical trees. Am. Nat. 187, 99–109 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Pérez-Harguindeguy N., et al. , New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234 (2013). [Google Scholar]
- 59.Coelho de Souza F., et al. , Evolutionary heritage influences Amazon tree ecology. Proc. R. Soc. B: Biol. Sci. 283, 20161587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laliberté E., Legendre P., Shipley B., FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
CWM trait data and trait range data of each plot have been deposited in the Data Archiving and Networked Services repository (https://doi.org/10.17026/dans-zz5-hf3s).