Abstract
Introduction: Characterizing patterns of laser activation is important for assessing thermal dose during laser lithotripsy. The objective of this study was twofold: first, to quantify the range of operator duty cycle (ODC) and pedal activation time during clinical laser lithotripsy procedures, and second, to determine thermal dose in an in vitro caliceal model when 1200 J of energy was applied with different patterns of 50% ODC for 60 seconds.
Methods: Data from laser logs of ureteroscopy cases performed over a 3-month period were used to calculate ODC (lasing time/lithotripsy time). Temporal and rolling 1-minute average power tracings were generated for each case. In vitro experiments were conducted using a 21 mm diameter glass bulb in a 37°C water bath, simulating a renal calix. A LithoVue ureteroscope with attached thermocouple was inserted and 8 mL/min irrigation was delivered with a 242 μm laser fiber within the working channel. In total, 1200 J of laser energy was applied in five different patterns at 20 W average power for 60 seconds. Thermal dose was calculated using the Sapareto and Dewey t43 method.
Results: A total of 63 clinical cases were included in the analysis. Mean ODC was 32% overall and 63% during the 1-minute of greatest energy delivery. Mean time of pedal activation was 3.6 seconds. In vitro studies revealed longer pedal activation times produced higher peak temperature and thermal dose. Thermal injury threshold was reached in 9 seconds when 40 W was applied at 50% ODC with laser activation patterns of 30 seconds on/off and 15 seconds on/off.
Conclusion: ODC was quantified from clinical laser lithotripsy cases: 32% overall and 63% during 1-minute of peak power. Time of pedal activation is an important factor contributing to fluid heating and thermal dose. Awareness of these concepts is necessary to reduce risk of thermal injury during laser lithotripsy procedures.
Keywords: laser lithotripsy, ureteroscopy, technique
Introduction
High-power Ho:YAG lasers (100–120 W) have widely expanded the available settings for laser lithotripsy and facilitated tailoring of treatment for individual cases. Additional modes of laser lithotripsy such as dusting and popcorning can be used in addition to traditional stone fragmentation to improve speed and efficiency of treatment and better control stone particle size. However, these newer modes of laser lithotripsy are commonly applied at higher pulse frequencies and with greater average power. This can result in excessive temperature elevation of the fluid within the collecting system as demonstrated in a number of in vitro and in vivo studies, as well as computer simulations.1–9
Many of the published studies assessing risk of thermal injury were designed with continuous laser activation of 1 minute or longer. This standardization is useful for scientific analysis and comparability between studies, but it is not directly applicable to clinical patterns of laser activation. In practice, the surgeon presses a foot pedal to activate the laser and thereby controls the time of each laser activation and the length of intervening pauses. Surprisingly, there is little in the literature describing patterns of laser activation for lithotripsy.
The concept of “operator duty cycle (ODC),” defined as “lasing time” divided by “lithotripsy time,” can be used to characterize laser activation during clinical cases. Lasing time is the summation of time the laser is activated (pedal depressed) and lithotripsy time is the time from first to last pedal activation including pauses (Table 1). For example, a urologist who activates the laser for 6 seconds, then pauses for 4 seconds would record a 60% ODC for this 10 seconds period. Managing the ODC during laser lithotripsy is one strategy to control thermal dose. The objective of this study was twofold: first, to quantify the range of ODC and pedal activation time during clinical laser lithotripsy procedures, and second, to determine the thermal dose produced in an in vitro caliceal model when 1200 J of laser energy was applied with different patterns of 50% ODC for 60 seconds.
Table 1.
Definitions of the Terms Used in This Study
| Term | Definition |
|---|---|
| Lasing time | Time laser is active (pedal depressed) |
| Lithotripsy time | Time from the first laser activation to the end of last laser activation including pedal on and off times |
| ODC | Lasing time/lithotripsy time |
ODC = operator duty cycle.
Methods
Evaluation of clinical cases
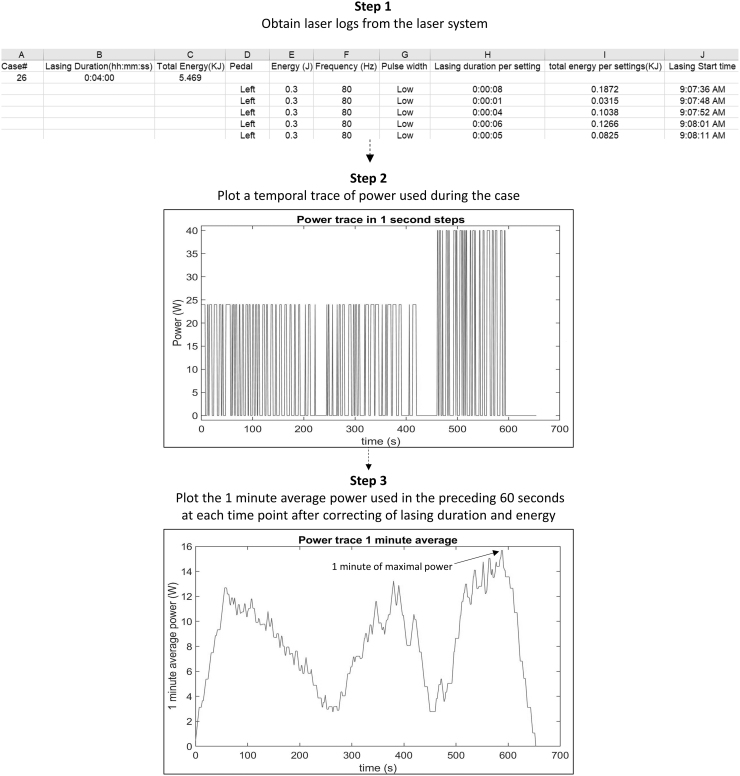
This study was reviewed and categorized as “not-regulated” by the institutional review board (IRB) at our institution. Laser lithotripsy cases performed by five endourology faculty between December 2018 and February 2019 and with total lasing time exceeding 3 minutes were included for analysis. Cases with at least 3 minutes of lasing time provided sufficient data to assess lithotripsy patterns. Deidentified data from each case were obtained from the laser log of clinical lasers (pulse 120; Lumenis, CA) including lasing time and energy for each case, as well as pulse energy, pulse frequency, and lasing time for each pedal activation (Fig. 1). Unfortunately, within the laser log, lasing time was rounded up to the next whole second. This necessitated calculation of a corrected lasing time for each pedal activation based on energy delivered and power selected. The corrected lasing time was calculated using the following formula: (lasing time = total energy delivered [J])/(pulse energy [J] × pulse frequency [Hz]). This corrected lasing time was used to calculate the ODC in this study.
FIG. 1.
Schematic of the steps used to calculate the ODC during the 1-minute period of greatest average power. ODC, operator duty cycle.
For each laser lithotripsy case, a temporal power trace (power [W] = pulse energy [J] × frequency [Hz]) was created using a customized Matlab algorithm (MathWorks, MA). From this power trace, a plot of the rolling 1-minute average power (average power applied in the preceding 1 minute) was derived. ODC was then calculated for the entire period of lithotripsy (time from first through the last activation of the laser) and during the 1-minute of greatest energy delivery for each case. Data were reported as mean and standard deviation.
In vitro caliceal model experiments
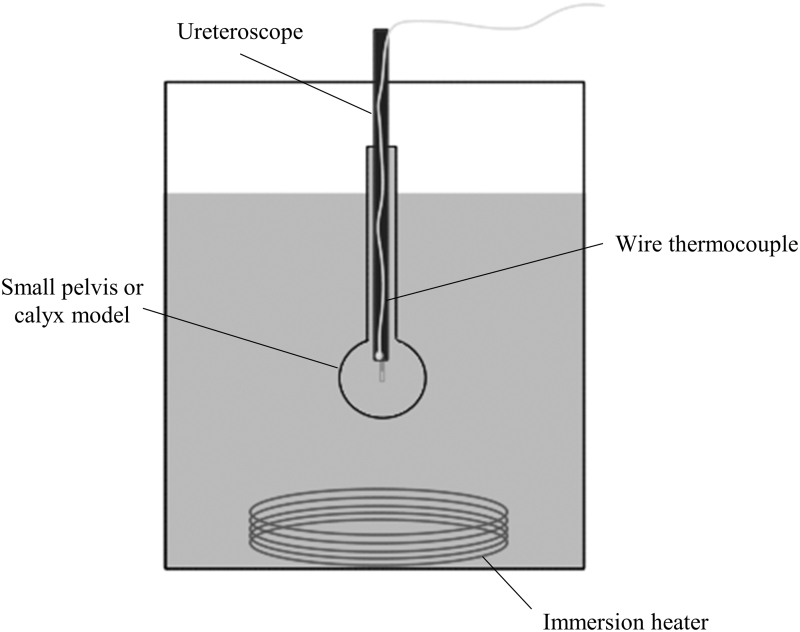
A cylindrical glass tube (inner diameter 6 mm, length 55 mm) with a glass bulb (19 mm inner diameter) at its distal end simulated a ureter and small renal pelvis or calix, respectively, for this experiment. This tube was positioned upright in a water bath maintained at 37°C ± 1°C by an immersion heater (Ulanet, CT) with its opening 1 cm above the water's surface. A flexible ureteroscope (LithoVue, Boston Scientific) was introduced into the glass tube through the upper opening and positioned so that its distal end was centered within the glass bulb. A 242 μm laser fiber (Flexiva, Boston Scientific) was then passed through the working channel of the ureteroscope, extending 5 mm beyond its distal tip. A wire thermocouple (Omega, CT) was secured adjacent to the ureteroscope (Fig. 2).
FIG. 2.
Experimental setup of the water bath maintained at 37°C showing the small pelvis or calix model and ureteroscope with the attached wire thermocouple.
Irrigation consisted of deionized water maintained at room temperature, 20°C ± 1°C, delivered to the ureteroscope from a peristaltic pump (Masterflex; Cole Parmer, IL) at 8 mL/min. Needle thermocouples (Physitemp, NJ) were used to record temperature every second within the irrigation reservoir and water bath.
The holmium:YAG laser (pulse120; Lumenis) was activated in short pulse mode to deliver 1200 J for 60 seconds in five different patterns: 0.5 J × 80 Hz (40 W) at 50% ODC (30 seconds on/30 seconds off), 0.5 J × 80 Hz (40 W) at 50% ODC (15 seconds on/15 seconds off × 2), 0.5 J × 80 Hz (40 W) at 50% ODC (10 seconds on/10 seconds off × 3), 0.5 J × 80 Hz (40 W) at 50% ODC (5 seconds on/5 seconds off × 6), and 0.5 J × 40 Hz (20 W) at 100% ODC. For each pattern, 1200 J was delivered with time averaged power of 20 W for 60 seconds. Three trials were performed for each pattern.
The mean and standard deviation of the fluid temperatures were calculated using Microsoft Excel (Redmond, WA) for each set of trials. Thermal dose was then calculated using the Sapareto and Dewey t43 methodology.10 A thermal dose of 120 equivalent minutes at 43°C was considered the threshold for thermal tissue injury.
Results
Evaluation of clinical cases
A total of 176 laser lithotripsy cases were performed during the study period of which 63 met the inclusion criteria; 113 cases were excluded because the total lasing time was <3 minutes.
For the remaining included cases, lithotripsy time averaged 23.2 minutes (±14.2; range 5.3–79.8 minutes) with lasing time of 6.6 minutes (±4.7; range 2.2–25.9 minutes). Mean ODC was 32% (±15%; range 6%–72%). The mean time of each pedal activation was 3.6 seconds (±3.0; range 0.1–182.0 seconds). During the 1-minute of greatest average power for each procedure (calculated by the method depicted in Fig. 1), the mean ODC was 63% (±21; range 12%–100%) and mean power was 18.5 W (±9.0; range 2.6–40.5 W).
In vitro caliceal model experiments
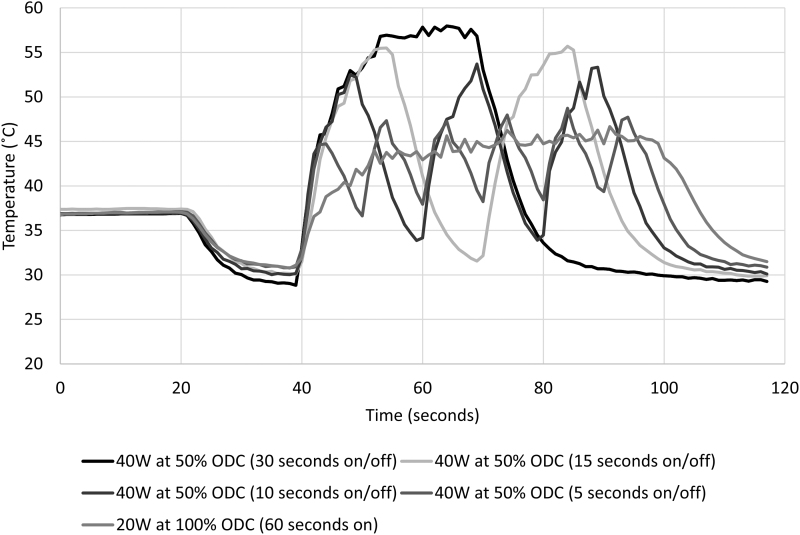
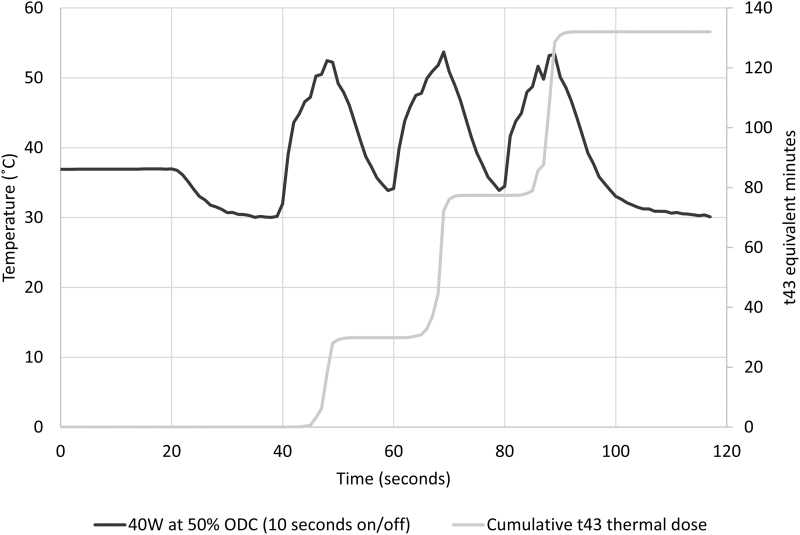
Equivalent energy (1200 J) was applied for all in vitro experiments at 50% ODC for 60 seconds. An immediate rise in fluid temperature was noted for all patterns of laser activation (Fig. 3). Temperature peaks, associated with each pedal activation, were greatest for the longest pedal activation and decreased with shortening of laser activation times (Fig. 4 and Table 2). The 50% ODC with longest pedal activation, (30 seconds on/30 seconds off) resulted in a thermal dose of 6813 equivalent minutes, the shortest pedal activation pattern tested (5 seconds on/5 seconds off × 6) resulted in a thermal dose of 6 equivalent minutes. As the pedal activation time is further shortened, a limit will be approached equivalent to 20 W applied at 100% ODC for 60 seconds, which resulted in a thermal dose of 4 equivalent minutes.
FIG. 3.
Temperature response from 1200 J laser energy delivered with different patterns and room temperature irrigation at 8 mL/min.
FIG. 4.
Caliceal fluid temperature and thermal dose (t43 equivalent minutes) from 1200 J laser energy delivered with 50% ODC and room temperature irrigation at 8 mL/min.
Table 2.
Time to Threshold of Thermal Injury and Thermal Dose for Different Patterns of Laser Activation and Room Temperature Irrigation at 8 mL/Min
| Pattern of laser activation for 60 seconds | Time to threshold of thermal injury (seconds) | t43 thermal dose (equivalent minutes) |
|---|---|---|
| 40 W applied with 50% ODC (30 seconds on/30 seconds off) | 9 | 6813 |
| 40 W applied with 50% ODC (15 seconds on/15 seconds off) × 2 | 9 | 1333 |
| 40 W applied with 50% ODC (10 seconds on/10 seconds off) × 3 | 45 | 158 |
| 40 W applied with 50% ODC (5 seconds on/5 seconds off) × 6 | — | 6 |
| 20 W applied with100% ODC | — | 4 |
The threshold of thermal injury, defined as t43 = 120 equivalent minutes, was reached after 9 seconds when the laser was activated at 40 W with 30 seconds on/off and 15 seconds on/off patterns (Table 2). For the activation pattern of 10 seconds on/off, threshold was reached in 45 seconds, during the third temperature peak. For shorter activation times, the threshold of injury was not reached, nor was it reached with 20 W at 100% ODC for 60 seconds.
Discussion
As laser systems of greater power are increasingly being utilized for laser lithotripsy, it is important that urologists develop a refined awareness of the temperature elevation and thermal dose that can result from high-power laser lithotripsy. It has been established that application of high-power laser energy can substantially increase fluid temperature within the collecting system and ureter when adequate irrigation is not achieved.1,2,6,8 This level of temperature elevation was correlated with histologic injury of the collecting system and adjacent parenchyma in a porcine model.2 These findings underscore the importance of developing strategies and techniques for predicting and controlling thermal dose to ensure patient safety when using high-power laser lithotripsy. This study sought to characterize laser activation patterns and their influence on thermal dose.
Evaluation of laser log data from clinical laser lithotripsy cases revealed an average ODC of 32% overall and 63% during the 1 minute of maximal energy delivery. Both measures help characterize patterns of laser activation. ODC during the 1-minute of maximal average power delivery is most relevant when assessing thermal dose since its contribution to thermal dose is far greater than from other time periods where temperatures are not as high.
“When considering temperature elevation within biologic systems, it is not adequate to define a threshold temperature for tissue injury and cellular death. Thermal tissue injury is dependent upon both the degree of temperature elevation and the duration of exposure to elevated temperatures.10 To standardize this concept for varying time–temperature curves, Dewey and Sapareto developed the concept of t43 equivalence. Using this methodology, any temperature curve can be converted into ‘equivalent minutes’ at 43°C. One minute of exposure to 43°C = 1 equivalent minute, at 44°C = 2 equivalent minutes, at 45°C = 4 equivalent minutes and so forth. The threshold for tissue injury and cell death varies between 120 and 240 minutes based on tissue type. In this study, 120 equivalent minutes was set as the thermal injury threshold. Understanding these concepts, one quickly sees that higher temperatures have a disproportionate effect on tissue toxicity. For example, tissue exposed for 1 minute at 49°C experiences a thermal dose of 64 equivalent minutes, whereas 1 minute of exposure to 56°C produces a thermal dose of 10,192 equivalent minutes.10,”11 Thermal dose is cumulative during the treatment period. Although temperature may return to baseline between laser activations, thermal dose does not return to baseline as shown in Figure 4.
Managing ODC to limit exposure to the highest temperatures is an important strategy for controlling thermal dose. From these in vitro experiments, it is also clear that the pattern in which this ODC is achieved is also important. Patterns with longer pedal activation result in higher thermal dose than patterns with shorter pedal activation such as 5 seconds on and 5 seconds off. Fortunately, from the analysis of clinical cases, the mean length of pedal activation was found to be relatively short—3.6 seconds. Although high-power laser techniques have clinical advantages,12,13 urologist need to be mindful of ODC and keep pedal activation times as short as possible.
Although the in vitro model used in this study does not fully replicate the in vivo environment, previous research demonstrated that it closely approximated the thermal measurements produced during in vivo porcine experiments.1,2 Second, energy was applied for only 30–60 seconds, which served to demonstrate the physical and thermal principles, but would be a gross underestimation of total energy applied and thermal dose achieved during many clinical laser lithotripsy cases. Third, the full parameter space was not explored—50% ODC was selected for in vitro studies as it fell within the relevant range utilized clinically. Evaluation of a broader range of ODC, pedal activation times, and irrigation rates is planned to further understand impact on thermal dose. In addition, incorporation of these metrics into previously published methods could further improve selection of thermally safe laser lithotripsy and irrigation parameters.14 Future study to characterize ODC when different pulse modes are selected may uncover pattern differences based on visualization or other factors specific to individual pulse modes.
Conclusions
This study determined ODC to be 32% overall and 63% during the 1 minute of greatest average power during clinical ureteroscopy cases. The time of continuous pedal activation was determined to be an important factor contributing to fluid heating during high-power laser lithotripsy; for delivery of equivalent energy, patterns with longer pedal activation times resulted in greater thermal dose. Awareness of these concepts, even generally, is important to influence lithotripsy techniques and reduce risk of thermal injury until temperature and cumulative thermal dose can be displayed in real time during laser lithotripsy procedures.
Abbreviations Used
- IRB
institutional review board
- ODC
operator duty cycle
Author Disclosure Statement
K.R.G. is a consultant for Lumenis, Boston Scientific, Olympus, and Coloplast. C.A.D. and W.W.R. are consultants for Boston Scientific.
Funding Information
J.M.H. received funding from NIDDK R01DK121709. This study was supported by a scientific research grant from Boston Scientific.
References
- 1. Aldoukhi AH, Ghani KR, Hall TL, Roberts WW. Thermal response to high-power holmium laser lithotripsy. J Endourol 2017;31:1308–1312. [DOI] [PubMed] [Google Scholar]
- 2. Aldoukhi AH, Hall TL, Ghani KR, Maxwell AD, MacConaghy B, Roberts WW. Caliceal fluid temperature during high-power holmium laser lithotripsy in an in vivo porcine model. J Endourol 2018;32:724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hein S, Petzold R, Schoenthaler M, Wetterauer U, Miernik A. Thermal effects of Ho: YAG laser lithotripsy: Real-time evaluation in an in vitro model. World J Urol 2018;36:1469–1475. [DOI] [PubMed] [Google Scholar]
- 4. Hein S, Petzold R, Suarez-Ibarrola R, Muller PF, Schoenthaler M, Miernik A. Thermal effects of Ho:YAG laser lithotripsy during retrograde intrarenal surgery and percutaneous nephrolithotomy in an ex vivo porcine kidney model. World J Urol 2020;38:753–760. [DOI] [PubMed] [Google Scholar]
- 5. Molina WR, Silva IN, Donalisio da Silva R, Gustafson D, Sehrt D, Kim FJ. Influence of saline on temperature profile of laser lithotripsy activation. J Endourol 2015;29:235–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sourial MW, Ebel J, Francois N, Box GN, Knudsen BE. Holmium-YAG laser: Impact of pulse energy and frequency on local fluid temperature in an in-vitro obstructed kidney calyx model. J Biomed Opt 2018;23:1–4. [DOI] [PubMed] [Google Scholar]
- 7. Winship B, Wollin D, Carlos E, et al. . The rise and fall of high temperatures during ureteroscopic holmium laser lithotripsy. J Endourol 2019;33:794–799. [DOI] [PubMed] [Google Scholar]
- 8. Wollin DA, Carlos EC, Tom WR, Simmons WN, Preminger GM, Lipkin ME. Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model. J Endourol 2018;32:59–63. [DOI] [PubMed] [Google Scholar]
- 9. Maxwell AD, MacConaghy B, Harper JD, Aldoukhi AH, Hall TL, Roberts WW. Simulation of laser lithotripsy-induced heating in the urinary tract. J Endourol 2019;33:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 1984;10:787–800. [DOI] [PubMed] [Google Scholar]
- 11. Dau JJ, Hall TL, Maxwell AD, Ghani KR, Roberts WW. Effect of chilled irrigation on caliceal fluid temperature and time to thermal injury threshold during laser lithotripsy: In vitro model. J Endourol 2020. [Epub ahead of print]; DOI: 10.1089/end.2020.0896. [DOI] [PubMed] [Google Scholar]
- 12. Pietropaolo A, Jones P, Whitehurst L, Somani BK. Role of “dusting and pop-dusting” using a high-powered (100 W) laser machine in the treatment of large stones (≥15 mm): Prospective outcomes over 16 months. Urolithiasis 2019;47:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tracey J, Gagin G, Morhardt D, Hollingsworth J, Ghani KR. Ureteroscopic high-frequency dusting utilizing a 120-W holmium laser. J Endourol 2018;32:290–295. [DOI] [PubMed] [Google Scholar]
- 14. Aldoukhi AH, Black KM, Hall TL, et al. . Defining thermally safe laser lithotripsy power and irrigation parameters: In vitro model. J Endourol 2020;34:76–81. [DOI] [PubMed] [Google Scholar]