Table 1.
SAR among representative class A’ analogs (1 – 19)
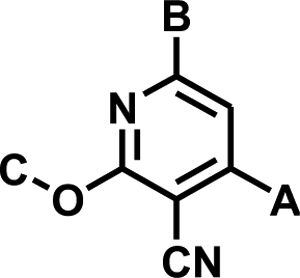
|
||||
|---|---|---|---|---|
| Compound | A | B | C | IC50 ± SE (nM) |
| 1 | 4-FPh | Ph | Me | 25 ± 2 |
| 2 | 4-FPh | 4-(MeO)Ph | Me | 1,360 ± 150 |
| 3 | 4-FPh | 3-(MeO)Ph | Me | 108 ± 6 |
| 4 | 4-FPh | 2-(MeO)Ph | Me | 75 ± 7.4 |
| 5 | 4-FPh | t-Bu | Me | 153 ± 14 |
| 6 | 4-ClPh | Ph | Me | 252 ± 21 |
| 7 | 2,4-Cl2Ph | Ph | Me | >100,000 |
| 8 | t-Bu | 2-Pyr | Me | 850 ± 140 |
| 9 | 4-FPh | Ph | MeO(CH2)2 | 123 ± 8.7 |
| 10 | Ph | Ph | PhCH2 | 100 ± 14 |
| 11 | 4-Pyr | Ph | Me | 541 ± 21 |
| 12 | 4-FPh | 3-Pyr | Me | 213 ± 24 |
| 13 | 4-FPh | 4-Pyr | Me | 17,400 ± 1300 |
| 14 | 4-FPh | 2-Pyr | Me | 14 ± 1.1 |
| 15 | 4-FPh | 3-Me-2-Pyr | Me | 8.7 ± 0.74 |
| 16 | 4-FPh | 2-Pyr | Allyl | 5.5 ± 0.39 |
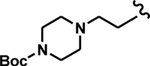
| 17 | 4-FPh | 3-Me-2-Pyr |
|
43 ± 2.4 |
| 18 | 4-FPh | 3-Me-2-Pyr | 3-PyrCH2 | 36 ± 3.1 |
| 19 | 4-NH2Ph | 3-Me-2-Pyr | Me | 29 ± 2.7 |
