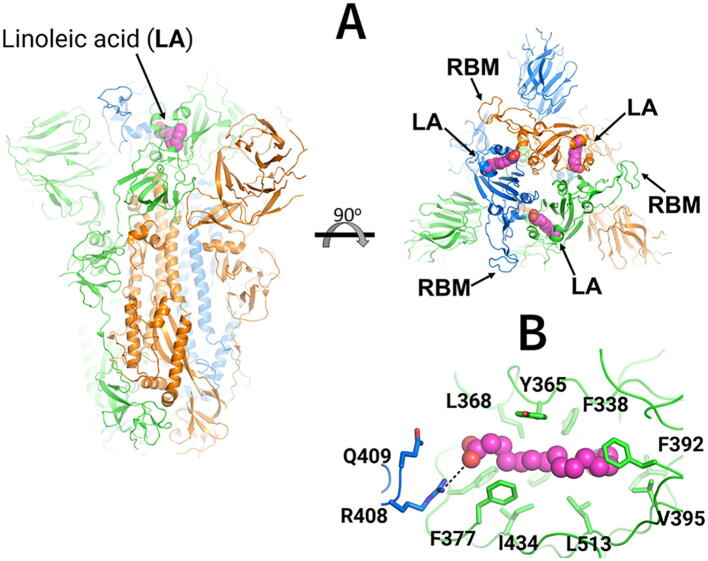
Fig. 1.
Cryo-EM structure of the ectodomain of the SARS-CoV-2 spike trimer with linoleic acid (LA) bound to the fatty acid-binding sites [19]. (A) Three-dimensional structure of the complex of the locked (in which all receptor-binding motifs (RBMs) are occluded) ectodomain of the SARS-CoV-2 spike trimer with linoleic acid (PDB code: 6ZB5) [19]. The spike protein is a homotrimer [18]: each monomer is shown in a different colour, namely green, orange and blue. LA molecules are highlighted with spheres. Each fatty acid (FA) binding site is located at the interface between two neighbouring monomers, and is formed by residues from two adjacent receptor-binding domains. (B) Detailed view of the FA binding site: this pocket is lined by hydrophobic and aromatic residues, and the LA acidic headgroup is close to R408 and Q409. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)