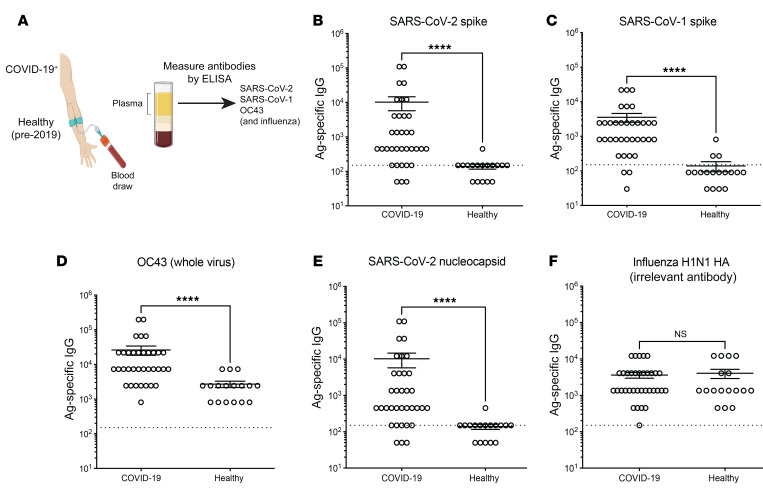
Figure 2. Cross-reactive antibody responses following SARS-CoV-2 infection in humans.
Antibody responses following SARS-CoV-2 infection. (A) Participants in the COVID-19 group had a positive RT-PCR test accompanied by mild to severe symptoms. Serum samples (35 COVID-19 and 17 healthy controls) were collected once from week 3 to week 45 following symptom onset for the COVID-19 cohort. The healthy control cohort refers to human plasma collected prior to 2019. (B) SARS-CoV-2 spike–specific antibody responses. (C) SARS-CoV-1 spike–specific antibody responses. (D) OC43-specific antibody responses. OC43-infected cell lysate was used as a coating antigen. (E) SARS-CoV-2 nucleocapsid–specific antibody responses. (F) Influenza virus H1N1 HA–specific antibodies. Antibody responses were evaluated by ELISA. Dashed lines represent the LOD. ****P < 0.0001, by nonparametric Mann-Whitney U test. Error bars indicate the SEM.