Abstract
Endothelial cell interactions with normal and cancerous breast epithelial cells have been widely studied in tissue growth and development, as well as in angiogenesis and metastasis. Despite the understanding that 3D multicellular architecture is critical to the cell phenotype, 3D vascular structures have not yet been cocultured with 3D breast spheroids in vitro. The objective of this study was therefore to create a hierarchical, multiscale model of vascular endothelial-breast epithelial cell interactions in which both cell types were assembled into their 3D architectures. The model was successfully fabricated by adding preformed breast spheroids onto preformed endothelial tube-like networks. Through this model, we observed that breast spheroids maintain vascular tube-like networks. Over time, breast epithelial cells migrate out of the spheroid structure along the endothelial networks. This research shows that 3D cell structures serve as an important building block for creating multicellular coculture models to study physiologically relevant cell–cell interactions.
Keywords: endothelial tubes, breast spheroids, growth factors, branching morphogenesis, shear stress, ErbB2
Graphical Abstract
INTRODUCTION
Both normal and cancerous breast tissues are multiscale hierarchical structures composed of a variety of individual cell types that assemble into larger 3D multicellular structures. These 3D multicellular structures then interact with surrounding structures and extracellular matrix through both biological and mechanical cues. 3D monoculture models have existed for decades in various forms and consistently show that both vascular endothelial and breast epithelial cells respond differently to environmental cues in a 3D environment than cells in traditional 2D culture.1–4 However, 3D coculture models are less common, largely due to the challenges in providing the necessary cues to simultaneous develop multiple cell types into their respective 3D architectures.
We are interested in studying the cross-talk that occurs between blood vessels and cancerous breast tissue. Much of our knowledge about the interactions between vascular endothelial and breast epithelial cells comes from 2D culture using conditioned medium, pseudo-3D culture in Transwell inserts, or 3D models in which the two cell types are encapsulated in separate gels, in which only one cell type is in its 3D architecture (e.g., endothelial tube-like networks cultured with breast epithelial cells) or in which breast epithelial cells are added to vascularized tissues.5–9 These coculture models provide limited physiological insight, especially since both cell types change their function when assembled into a 3D architecture more similar to the in vivo tissue. In addition, cell–cell contact has recently been shown to be critical to blood vessel–breast tissue interactions.10–12
The objective of this study was therefore to create a coculture model of vascular endothelial–breast epithelial cell interactions in which both cell types were assembled into their 3D architecture. Since cocultures of the two cell types did not spontaneously form either endothelial tube-like networks or breast epithelial cell spheroids, we created a 3D coculture model by adding preformed breast epithelial cell spheroids onto preformed endothelial tube-like networks. This method preserved the viability and structure of both cell types and enabled us to observe cell–cell interactions that relied on both secreted factors and cell–cell contact. This research shows that 3D cell structures provide an important building block for creating multicellular coculture models to study physiologically relevant cell–cell interactions.
MATERIALS AND METHODS
Cell Culture.
The breast cancer cell lines MCF-10A and MCF10A-NeuN, which stably express human ErbB2, were a generous gift from Dr. Mauricio Reginato (Drexel College of Medicine).13,14 MCF10A and MCF10A-NeuN cells were cultured in growth medium which consisted of DMEM/F12 (Cellgro, Manassas,VA), 5% horse serum (Invitrogen, Waltham, MA), 20 ng/mL epidermal growth factor (EGF; Peprotech, Rocky Hill, NJ), 10 μg/mL bovine insulin (Sigma, St. Louis, MO), 10 ng/mL cholera toxin (Enzo Life Sciences, Farmingdale, NY), 500 ng/mL hydrocortisone (Sigma), and 1% penicillin-streptomycin (Invitrogen, Waltham, MA). Human umbilical vein endothelial cells (HUVEC, Cell Applications) were maintained in Endothelial Growth Medium-2 (EGM-2; Lonza, Allendale, NJ) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 1% penicillin-streptomycin, and 1% L-glutamine (Invitrogen, Waltham, MA). All cells were maintained in a humidified environment at 37 °C and 5% CO2, with a medium change every 2 days. Breast cell lines were used up to passage 25, and HUVEC were used up to passage 8.
3D Breast Spheroid Formation.
To grow 3D breast spheroids, 30 μL of growth factor reduced Matrigel (Corning, Manassas, VA) was added to each well of an eight-chamber polystyrene vessel affixed to a tissue culture treated glass slide (BioCoat Falcon Culture slides, Corning, Bedford, MA) and incubated at 37 °C for 20 min to solidify the Matrigel. MCF10A or MCF10A-NeuN cells were trypsinized and resuspended in growth medium with 20% Matrigel (3D medium). A total of 4000 cells were added to each chamber well. MCF10A cells formed spheroids in 8–10 days, whereas MCF10A-NeuN cells formed spheroids within 5–6 days. Medium was replaced every 4 days.
Endothelial Network Formation.
To create endothelial tube-like structures, Matrigel was prepared as for the 3D breast spheroids. HUVEC were trypsinized, resuspended in Endothelial Basal Medium-2 (EBM-2; Lonza), and labeled with CellTracker Red (1:1000; Invitrogen) as per manufacturer instructions. A total of 20000 HUVEC were then added to each well in serum-free EBM-2 and left undisturbed in a 37 °C, 5% CO2 incubator for 6 h to form networks.
2D HUVEC Coculture with Breast Epithelial Cells.
For 2D coculture experiments, 10000 MCF10 or MCF10A-NeuN cells were seeded on confluent CellTracker (Invitrogen) labeled HUVEC monolayers, with monocultures as controls. After 24 h, cells were fixed in 4% paraformaldehyde and prepared for confocal microscopy as described below. 2D cocultures were also monitored over time using the IncuCyte Live Cell Analysis System (Essen Bioscience, Ann Arbor, MI). To fluorescently distinguish the two different cell types, HUVEC were stably transfected to label mitochondria with green fluorescent protein (GFP-HUVEC; virus kindly provided by Dr. Christian Sells, Drexel College of Medicine) or the MDA-MB-231 breast cancer cell line was stably transfected with red fluorescent protein using Nuclight Red (RFP-MDA-MB-231; Essen Bioscience). Samples included (1) confluent GFP-HUVEC; (2) 10000 RFP-MDA-MB-231; and (3) confluent GFP-HUVEC with 10000 RFP-MDA-MB-231. In addition, HUVEC death was measured over time with a cytotoxicity assay. Samples included (1) confluent HUVEC; (2) 10000 MDA-MB-231; and (3) confluent HUVEC with 10000 MDA-MB-231. Cytotox Green (Essen Bioscience) was added to these samples to quantify dead cells. Samples were imaged every 4 h for 3 days. Images were analyzed using Incucyte Zoom software.
HUVEC Network Coculture with 3D Breast Epithelial Spheroids.
For 3D coculture experiments, preformed breast spheroids were added to preformed endothelial networks. Specifically, breast spheroids were carefully pipetted out of their wells using a 1 mL pipet with 0.5 cm cut off the pipet tip end. HUVEC networks were washed twice with phosphate buffered saline (PBS) to remove excess CellTracker. Spheroids were then pipetted onto the HUVEC networks, with one well of spheroids split into two wells of HUVEC networks to decrease spheroid aggregation. The coculture was maintained in 3D medium for 24–96 h.
Confocal Microscopy.
To prepare samples for confocal microscopy, mono- and cocultures were fixed with 4% paraformaldehyde for 1 h at room temperature. Samples were then blocked with Immunofluorescence (IF) wash (0.05% w/v NaN3, 0.1% w/v BSA, 0.2% v/v Triton-X 100, 0.04% v/v Tween-20 in PBS, pH 7.4) with 10% goat serum for 90 min (primary block), followed by IF wash with 10% goat serum and F(ab′)2 fragment goat antimouse IgG (1:100; Jackson ImmunoResearch, West Grove, PA; secondary block). Samples were then incubated with a primary antibody for integrin α6 (1:100, Millipore, Billerica, MA) overnight at 4 °C, followed by an Alexa Fluor 488 secondary antibody (1:200; Invitrogen) and Hoescht 33342 (10 μg/mL, Thermo Scientific, Waltham, MA) for 1 h at room temperature, protected from light. After thorough washing, samples were carefully detached from the slides and mounted on coverslips in PBS with 0.75% w/v glycine. Samples were then imaged using either an LSM 700 (Zeiss, Thornwood, NY) or FV1000 (Olympus, Waltham, MA) confocal microscope.
Breast Epithelial Spheroid Conditioned Media on HUVEC.
MCF10A and MCF10A-NeuN cell conditioned media was added to HUVEC networks to determine if coculture effects were mediated through breast cell-secreted soluble factors. To collect conditioned media, MCF10A and MCF10A-NeuN cells were cultured to form spheroids as described. Fresh 3D medium was then added to MCF10A spheroids (at 7 days) or MCF10A-NeuN spheroids (at 4 days) for 24 h. 3D medium was also added to empty wells as a control. After 24 h, conditioned medium was removed from each well, centrifuged at 3000 rpm for 5 min to pellet debris, and then the supernatant was transferred into a new tube. Conditioned medium was mixed 1:1 with fresh 3D medium and added to preformed HUVEC networks. For some samples, a VEGF neutralizing antibody (1 μg/mL; R&D systems, Minneapolis, MN) was preincubated with the conditioned media for 1 h at 37 °C to bind any VEGF in the breast epithelial cell conditioned medium. The medium with neutralized VEGF was then added to HUVEC tubes as described. A total of 10 ng/mL vascular endothelial growth factor (VEGF, Peprotech) was the positive control. Samples were imaged by phase contrast microscopy (Eclipse TS100, Nikon Microscopes, Inc., Melville, NY) after 24 and 48 h and analyzed using the ImageJ angiogenesis plug in.
HUVEC Conditioned Media on 3D Breast Epithelial Spheroids.
HUVEC tube conditioned media was added to MCF10A and MCF10A-NeuN spheroids to determine if coculture effects were mediated through HUVEC-secreted soluble factors. Conditioned media was also collected from confluent HUVEC monolayers in static culture or exposed to steady laminar or oscillating disturbed flow for 24 h, with an empty dish of EGM-2 with 10% FBS as the control. HUVEC conditioned medium was then mixed 1:1 with 3D medium and added to fully formed MCF10A (day 8) or MCF10A-NeuN spheroids (day 5) for 24 h, with EGF (10 ng/mL) as the positive control. An EGF neutralizing antibody was incubated with HUVEC conditioned medium samples for 1 h at 37 °C to bind any EGF in the endothelial cell conditioned medium. The medium with neutralized EGF was then added to breast spheroids. Samples were then fixed with 4% paraformaldehyde, labeled, and imaged by confocal microscopy as described.
Statistics.
All statistical analyses were conducted using GraphPad Prism software. Graphs represent mean ± standard deviation. Multiple groups were compared using either two-way or n-way ANOVA with posthoc Tukey-Kramer test, and two groups were compared by Student’s t test. All experiments were conducted at least two times, with at least three samples per experiment.
RESULTS
Breast Epithelial Cells Could Not Be Cultured on Top of a Living Endothelial Cell Monolayer.
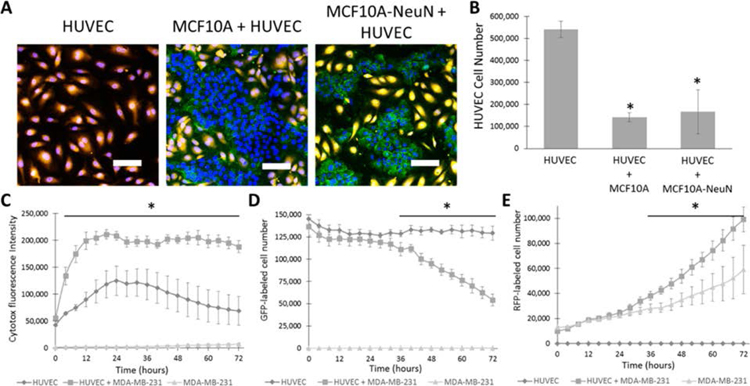
We first attempted to create a vascular endothelial cell–breast epithelial cell coculture system by seeding a small number of MCF10A (noncancerous) or MCF10A-NeuN (ErbB2 overexpressed) cells onto a fluorescently tagged HUVEC monolayer. After 24 h, both breast cell lines eliminated the HUVECs that were underneath them (Figure 1A). Viable HUVECs decreased by more than 4-fold when HUVECs were cocultured with breast cells, as compared to HUVECs in monoculture (Figure 1B). This decrease in cell number shows that viable endothelial cells cannot be maintained underneath breast epithelial cells.
Figure 1.
Breast epithelial cell coculture on top of an endothelial monolayer decreased endothelial cell number. (A) 10000 MCF10A or MCF10A-NeuN cells were seeded on confluent HUVEC monolayers (orange). After 24 h, cells were fixed, labeled for nuclei (blue, endothelial and breast cells) and integrin α6 (green, breast cells), and imaged by confocal microscopy. Representative confocal microscopy images and (B) HUVEC cell number quantification. (C) HUVEC monolayer cocultured with 10000 MDA-MB-231 cells was labeled with a cytotoxicity indicator and imaged over time in an Incucyte Live Cell Analysis System. (D) GFP-HUVEC cell number in coculture with RFP-MDA-MB-231 cell number, and (E) RFP-MDA-MB-231 in coculture with GFP-HUVEC. *p < 0.01 compared to HUVEC or MDA-MB-231 alone. Scale bar: 20 μm.
To determine if endothelial cell number stabilized after 24 h, as well as to ensure that this effect also occurred with more commonly used breast cancer cell lines, we measured cytotoxicity as well as total endothelial and breast cell number over time with the MDA-MB-231 (triple-negative cancerous) cell line. In the cytotoxicity assay, HUVEC monolayers alone showed significant cytotoxicity within 24 h of the start of the assay, and the cytotoxicity labeling decreased to baseline by 72 h (Figure 1C). HUVEC monolayers cocultured with a small number of MDA-MB-231 cells showed nearly twice as much cytotoxicity as HUVEC monolayers alone at 24 h, and the cytotoxicity labeling remained constant up to 72 h. To support these data and test a common breast cancer cell line, we then tracked GFP-HUVECs (labeled mitochondria) and RFP-MDA-MB-231 cells (labeled nuclei) over time in mono- and coculture. When GFP-HUVEC were cultured alone, GFP-labeled cell number remained relatively stable over 72 h (Figure 1D). For GFP-HUVEC cultured with MDA-MB-231 cells, GFP-labeled cell number decreased after 24 h and continued to decline up to 72 h. By 72 h, GFP-labeled cells in coculture were less than half of the monoculture. Interestingly, RFP-labeled cell number was higher in MDA-MB-231 cells cocultured with HUVECs than for cells in monoculture, an effect which started around the same time that HUVEC number decreased and continued to 72 h (Figure 1E). These data suggest that breast epithelial cells either crowd out or cause endothelial cell death in coculture, and we could not coculture breast epithelial cells on top of living endothelial cells. We therefore assessed alternative coculture methods using 3D cell building blocks.
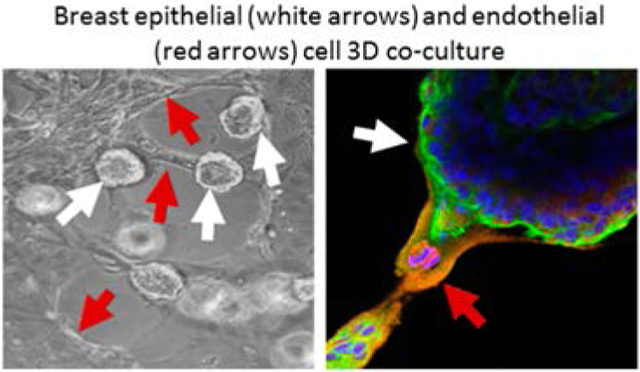
3D Endothelial Tube-Like Networks Can Be Cocultured with 3D Breast Spheroids.
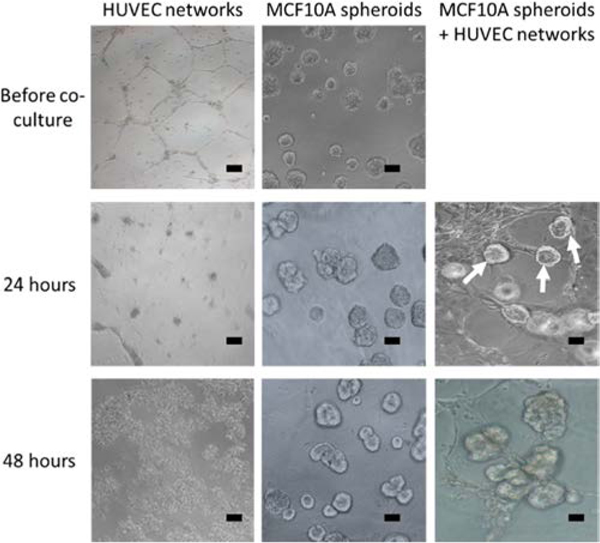
Since directly coculturing endothelial and breast cells failed in 2D, we explored whether 3D culture might preserve the two cell types. Initial attempts, including culturing HUVECs in Matrigel during 3D breast spheroid formation or adding HUVECs to preformed 3D breast spheroids in Matrigel, were not successful (data not shown). Instead, we found that both cell types survived and interacted when preformed 3D breast spheroids were added to preformed HUVEC tube-like networks (Figure 2). HUVEC networks formed in 6–8 h of serum-free culture on Matrigel. When these networks were then maintained in 3D medium, they completely collapsed within 24 h, and the HUVEC detached from the Matrigel surface after 48 h (Figure 2). In contrast, when 3D breast spheroids were added on to the HUVEC tube-like networks, the networks were still maintained even after 48 h of coculture. In addition, the 3D breast spheroids appeared to preferentially attach to the HUVEC tube-like structures (white arrows in Figure 2). We therefore further investigated the mutual interactions between HUVEC tube-like networks and 3D breast spheroids in coculture.
Figure 2.
Preformed 3D breast spheroids (white arrows) preferentially attached to preformed endothelial tube-like networks and maintain network viability. Samples were imaged by phase contrast microscopy. Scale bar: 20 μm.
Breast Spheroid Conditioned Media Maintains HUVEC Tube-Like Networks Likely through VEGF.
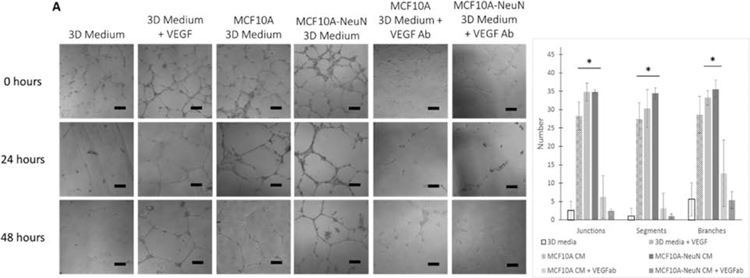
We first examined whether the 3D breast spheroids maintained the HUVEC tube-like networks through physical contact or a secreted factor by adding breast spheroid conditioned 3D medium to preformed HUVEC networks in a 1:1 ratio with EBM-2. HUVEC networks cultured with 1:1 3D medium and EBM-2 collapsed within 24 h (Figure 3A). When 10 ng/mL VEGF was added to this medium as a positive control, HUVEC networks were maintained for 48 h. Similarly, both MCF10A and MCF10A-NeuN conditioned 3D medium maintained the HUVEC networks. However, when VEGF in the 3D breast spheroid conditioned medium was neutralized, HUVEC networks again collapsed within 24 h. The ImageJ angiogenesis analyzer was used to quantify network junctions, segments, and branches across all samples (Figure 3B). HUVEC network junctions, segments, and branches were all increased by breast spheroid conditioned 3D medium. This effect was abrogated by neutralizing VEGF. Similar results were obtained for MDA-MB-231 breast epithelial spheroids (data not shown). These results suggest that both noncancerous and cancerous breast epithelial cells secrete VEGF, which maintains HUVEC networks over time.
Figure 3.
Breast spheroid conditioned media (CM) sustained HUVEC networks, likely through VEGF. (A) Representative phase contrast images. (B) ImageJ quantification of junctions, branches, and segments after 48 h. Scale bar = 50 μm. *p < 0.01 compared to 3D medium.
3D Breast Epithelial Cell Spheroids Elongate Along HUVEC Networks.
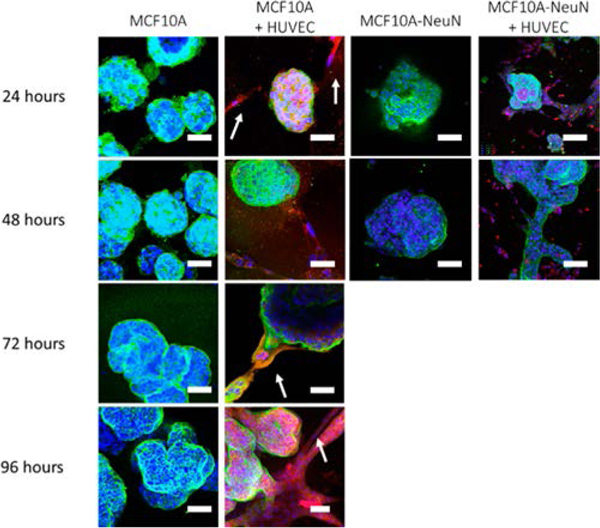
Since we observed that 3D breast epithelial cell spheroids preferentially attached to HUVEC tube-like networks in coculture, we examined 3D breast spheroid morphology in coculture over time. We added preformed 3D breast spheroids (formed over 5–8 days) to preformed HUVEC tube-like networks (formed over 6–8 h). In monoculture, MCF10A 3D spheroids that had been formed over 8 days began to cluster into larger, irregular aggregates with a blebbed morphology after an additional 72 h of culture (Figure 4). When preformed MCF10A spheroids were added to HUVEC networks, the spheroids primarily attached to the HUVEC tube-like networks. After 72 h of coculture, the MCF10A spheroids began to elongate along the HUVEC structures, and by 96 h, the spheroids completely covered the HUVEC networks (white arrows). Interestingly, the preformed MCF10A-NeuN 3D spheroids (5 days) elongated along the HUVEC tube-like networks within only 24 h of coculture (Figure 4, see also Supporting Information, Movies 1 and 2). These data suggest that HUVECs alter 3D breast spheroid morphology perhaps through an ErbB2-mediated mechanism.
Figure 4.
MCF10A spheroids elongated along HUVEC tube-like networks in 72–96 h, whereas MCF10A-NeuN spheroids elongated within 24 h. Preformed breast spheroids were added to preformed endothelial tube-like networks labeled with CellTracker (red). After the incubation period, samples were fixed and labeled for integrin α6 (green) and Hoechst (blue). Representative confocal microscopy images. Scale bar: 20 μm.
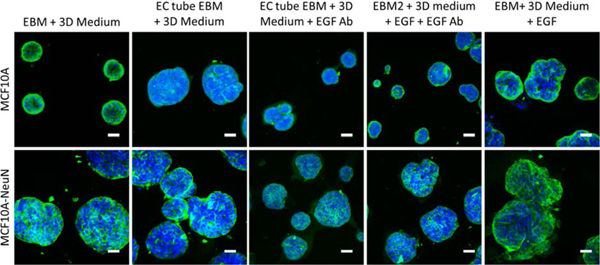
HUVEC Alter Breast Spheroid Morphology Likely through EGF.
Finally, 3D breast epithelial spheroids were cultured with conditioned medium from HUVEC tubes to determine if a HUVEC-secreted factor led to the morphological change (Figure 5). EBM alone did not change breast spheroid morphology; however EBM that had been conditioned with HUVEC tubes appeared to cause more blebs on the MCF10A spheroids and cause some MCF10A-NeuN cells to migrate out of their spheroids. This effect was similar to that observed in the EGF positive control. When EGF was blocked with a neutralizing antibody, the change in breast spheroid shape with endothelial tube conditioned media decreased.
Figure 5.
HUVEC tube conditioned media caused breast spheroids to change their morphology. Preformed MCF10A or MCF10A-NeuN breast cell spheroids were incubated with conditioned media collected from HUVEC tube-like networks. In some conditioned media samples, EGF (20 ng/mL) was added as a positive control, or a neutralizing EGF antibody (0.1 μg/mL) was preincubated with conditioned media samples prior to adding media to breast spheroids. Samples were then fixed, labeled, and imaged by confocal microscopy after 24 h. (Integrin α6 = green and nuclei = blue). Scale bar: 50 μm.
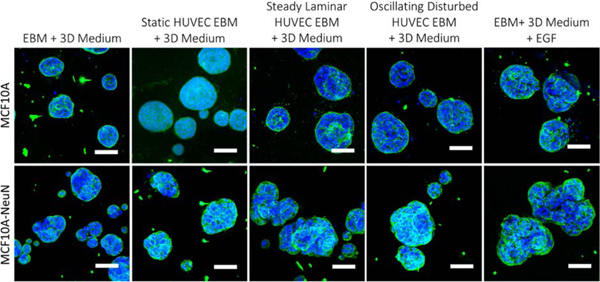
We also cultured 3D breast epithelial spheroids with conditioned medium from endothelial cells in static culture or exposed to steady laminar or oscillating disturbed flow, since our long-term goal is to include physiological fluid flow through endothelial tubes in the coculture model (Figure 6). Steady laminar and oscillating disturbed flow change many aspects of endothelial phenotype through varied mechanisms;15 however, flow effects on endothelial cell interactions with breast epithelial cells have not been examined. MCF10A and MCF10A-NeuN breast spheroids incubated with EBM-2 from HUVECs in static culture as well as from HUVECs exposed to steady laminar or oscillating disturbed flow appeared to have more blebs and areas in which breast epithelial cells were leaving the spheroids. These results were similar to spheroids cultured with EGF, and the effects were partially abrogated by preincubating the endothelial cell conditioned medium with an EGF neutralizing antibody.
Figure 6.
HUVEC conditioned media from cells exposed to laminar flow or with EGF caused MCF10A-NeuN spheroids to change their morphology. Preformed MCF10A or MCF10A-NeuN breast cell spheroids were incubated with HUVEC conditioned media, fixed, labeled, and imaged by confocal microscopy after 48 h. (Integrin α6 = green and nuclei = blue). Scale bar: 20 μm.
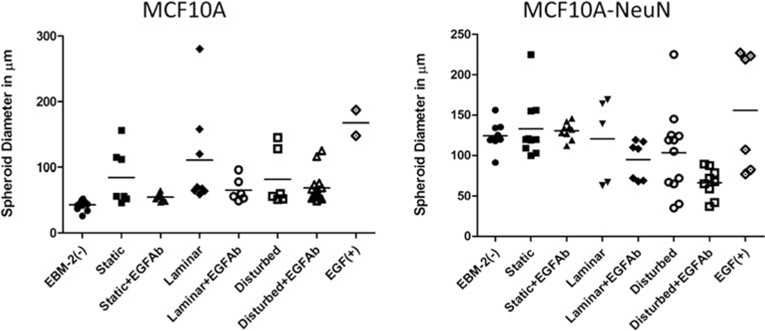
Finally, we quantified breast spheroid size in conditioned media experiments by measuring the largest dimension across each nonclustered spheroid (Figure 7). MCF10A-NeuN spheroids were on average larger than MCF10A spheroids in all conditions. We also observed more size variability for both MCF10A and MCF10A-NeuN spheroids when they were cultured in endothelial cell conditioned medium. This variability was diminished when the EGF neutralizing antibody was added. Although more studies are needed to determine the mechanism underlying this effect, the data suggest that endothelial cells may secrete EGF which causes spheroids to change their size and shape in a heterogeneous manner.
Figure 7.
MCF10A spheroids were smaller than MCF10A-NeuN spheroids. All spheroids cultured in endothelial cell conditioned media showed increased size variability, which was abrogated with an EGF neutralizing antibody. MCF10A or MCF10A-NeuN breast spheroids that had been incubated with HUVEC conditioned media as described were measured at their largest dimension in ImageJ.
DISCUSSION
Hierarchical, multicellular models of healthy and diseased tissue are essential to taking advantage of both the high experimental control of in vitro systems and the physiological relevance of in vivo studies. While both endothelial cells and breast epithelial cells can be cultured in vitro with their characteristic 3D in vivo architecture, these two cell types had not yet been integrated into a 3D coculture model of vascular–breast tissue interactions. We now show that preformed breast epithelial spheroids can be added to preformed endothelial tube-like networks and cultured over several days to observe, measure, and manipulate endothelial–epithelial cell interactions. We further demonstrate that both noncancerous and cancerous breast spheroids maintain HUVEC tube-like networks, likely through VEGF. In addition, breast epithelial cells migrated out of their spheroids and along HUVEC networks, which appeared to be partially mediated through secreted EGF and partially through cell–cell contact. These results demonstrate that this model produces unique interactions between these two cell types and may be useful in expanding our understanding of the vasculature in normal and cancerous tissues.
Many studies have previously shown that breast cancer cells induce endothelial angiogenesis, largely through VEGF secretion.16 More recently, it has been shown that endothelial cells may actively regulate tumor cell angiogenic factor expression.17,18 Our data suggest that nontumorous breast epithelial cells also secrete VEGF, and that this VEGF likely supports endothelial tube-like network maintenance. However, it is also possible that another biochemical factor in the breast epithelial cell conditioned medium causes the endothelial cells to increase VEGF secretion, which then maintains the HUVEC tubes. Interestingly, breast epithelial cells only supported endothelial cell survival in 3D culture. It is possible that breast epithelial cells do not secrete VEGF in 2D coculture with endothelial cells, or that the VEGF they secrete is not adequate to counteract the cell death signals in 2D contact coculture. In addition, endothelial cell VEGF response, among many other cell functions, is different in 2D vs 3D culture.19 While a wide variety of molecular mechanisms could cause this effect, one possibility is VEGF receptor-integrin colocalization.20 It would further be interesting to examine in our model if nontumorous breast epithelial cell VEGF secretion is modulated by endothelial cell coculture, or if these interactions are changed in 3D vs 2D culture. In addition, alternate scaffold materials with increased or decreased growth factor binding affinity could clarify the role that extracellular matrix composition plays in creating and sustaining the temporal and spatial growth factor gradients needed for efficient angiogenesis.
Our data also show that endothelial cell coculture stimulates breast epithelial cell migration out of spheroids and along endothelial tube-like networks. One possible factor may be EGF secretion by endothelial cells. EGF has been shown to induce EGFR-expressing cancer cells to convert from an epithelial to mesenchymal phenotype, termed epithelial-to-mesenchymal transition (EMT).21 Endothelial cell-secreted EGF in conditioned medium induced squamous cell carcinoma EMT in vitro, and tumor xenografts which contained EGF-silenced endothelial cells had fewer cancer-like stem cells in vivo.22 We demonstrated that MCF10A-NeuN breast epithelial cells migrated out of the spheroids and along the endothelial networks more quickly than the MCF10A cells. The MCF10A-NeuN cell overexpress ErbB2/HER2, a member of the EGFR family that is a preferred heterodimerization partner for EGFR among others.23 The rapid migration of these cells out of the spheroids may relate to direct EGFR interactions with ErbB2/HER2, or may be mediated through other factors such as prostate derived Ets factor (PDEF).24
In addition, conditioned media from endothelial cells induced morphological changes in MCF10A and MCF10A-NeuN spheroids. Laminar flow was previously shown to increase EGF mRNA in HUVEC within 3 h.25 These data suggest that the 20–30% of breast cancers that overexpress HER2 and have a poor prognosis due to high incidence of metastasis and chemotherapy resistance may be more sensitive to endothelial cell interactions.26 However, the effects of endothelial network coculture on breast spheroid morphology were not closely recapitulated by conditioned medium alone. The endothelial cell networks themselves may guide breast epithelial cell migration, possibly through binding of epithelial cells to cadherins, integrins, or matrix proteins on the endothelial apical surface. This migration mechanism may be similar to how collagen fibers orient the migrating mammary epithelium.27
The current model supports the idea that preformed 3D cell structures can successfully be used for creating multicellular models. In the future, we plan to enhance our model by adding a perfused vascular network so that we can study the effects of blood flow rate. We also hope to use tissue derived 3D cell structures, such as mammary organoids and adipose tissue microvascular fragments, which bring with them additional stromal cells (e.g., pericytes, fibroblasts) and tissue-specific extracellular matrix.28,29 While the vast majority of studies on endothelial–breast epithelial cell interactions have focused on angiogenesis and metastasis, these two cell types are likely to have many other significant interactions. In future work, we plan to measure specific cell functions, including glucose metabolism, to determine if perfused 3D engineered in vitro coculture models can shed light into tumor metabolic heterogeneity.30
CONCLUSIONS
3D in vitro multicellular models can be successfully fabricated from 3D cellular structures and demonstrate unique cell–cell interactions.
Supplementary Material
Movie 1: MCF10A-green_HUVEC-red.mpg shows MCF10A breast epithelial cell spheroids (green) cultured on a HUVEC layer (red) for 24 h (MPG).
Movie 2: MCF10A-NeuN-red_HUVEC-green.mpg shows MCF10A-NeuN breast epithelial cell spheroids (red) cultured on a HUVEC layer (green) for 24 h (MPG).
ACKNOWLEDGMENTS
We thank Mauricio Reginato and Christian Sells for providing transfected cells and reagents, as well as protocols and critical advice, for 2D and 3D cultures.
Footnotes
Notes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsbiomaterials.6b00624.
REFERENCES
- (1).Schmeichel KL; Bissell MJ Modeling tissue-specific signaling and organ function in three dimensions. J. Cell Sci 2003, 116 (12), 2377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Weigelt B; Ghajar CM; Bissell MJ The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Adv. Drug Delivery Rev 2014, 69–70, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Koh W; Stratman AN; Sacharidou A; Davis GE In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008, 443, 83–101. [DOI] [PubMed] [Google Scholar]
- (4).Sacharidou A; Koh W; Stratman AN; Mayo AM; Fisher KE; Davis GE Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood 2010, 115 (25), 5259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Buchanan CF; Szot CS; Wilson TD; Akman S; Metheny-Barlow LJ; Robertson JL; Freeman JW; Rylander MN Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. J. Cell. Biochem 2012, 113 (4), 1142–51. [DOI] [PubMed] [Google Scholar]
- (6).Szot CS; Buchanan CF; Freeman JW; Rylander MN In vitro angiogenesis induced by tumor-endothelial cell co-culture in bilayered, collagen I hydrogel bioengineered tumors. Tissue Eng., Part C 2013, 19 (11), 864–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Franses JW; Baker AB; Chitalia VC; Edelman ER Stromal endothelial cells directly influence cancer progression. Sci. Transl. Med 2011, 3 (66), 66ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Franses JW; Drosu NC; Gibson WJ; Chitalia VC; Edelman ER Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int. J. Cancer 2013, 133 (6), 1334–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Phamduy TB; Sweat RS; Azimi MS; Burow ME; Murfee WL; Chrisey DB Printing cancer cells into intact microvascular networks: a model for investigating cancer cell dynamics during angiogenesis. Integr Biol. (Camb) 2015, 7 (9), 1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Connor Y; Tekleab S; Nandakumar S; Walls C; Tekleab Y; Husain A; Gadish O; Sabbisetti V; Kaushik S; Sehrawat S; Kulkarni A; Dvorak H; Zetter B; R. E E; Sengupta S Physical nanoscale conduit-mediated communication between tumour cells and the endothelium modulates endothelial phenotype. Nat. Commun 2015, 6, 8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ghajar CM; Peinado H; Mori H; Matei IR; Evason KJ; Brazier H; Almeida D; Koller A; Hajjar KA; Stainier DY; Chen EI; Lyden D; Bissell MJ The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol 2013, 15 (7), 807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Strilic B; Yang L; Albarran-Juarez J; Wachsmuth L; Han K; Muller UC; Pasparakis M; Offermanns S Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 2016, 536 (7615), 215–8. [DOI] [PubMed] [Google Scholar]
- (13).Haenssen KK; Caldwell SA; Shahriari KS; Jackson SR; Whelan KA; Klein-Szanto AJ; Reginato MJ ErbB2 requires integrin alpha5 for anoikis resistance via Src regulation of receptor activity in human mammary epithelial cells. J. Cell Sci 2010, 123 (Pt 8), 1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Tchafa AM; Ta M; Reginato MJ; Shieh AC EMT Transition Alters Interstitial Fluid Flow-Induced Signaling in ERBB2-Positive Breast Cancer Cells. Mol. Cancer Res 2015, 13 (4), 755–64. [DOI] [PubMed] [Google Scholar]
- (15).Chiu JJ; Chien S Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev 2011, 91 (1), 327–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Carmeliet P; Jain RK Angiogenesis in cancer and other diseases. Nature 2000, 407 (6801), 249–57. [DOI] [PubMed] [Google Scholar]
- (17).Kaneko T; Zhang Z; Mantellini MG; Karl E; Zeitlin B; Verhaegen M; Soengas MS; Lingen M; Strieter RM; Nunez G; Nor JE Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007, 67 (20), 9685–93. [DOI] [PubMed] [Google Scholar]
- (18).Butler JM; Kobayashi H; Rafii S Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat. Rev. Cancer 2010, 10 (2), 138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Bowers SL; Norden PR; Davis GE Molecular Signaling Pathways Controlling Vascular Tube Morphogenesis and Pericyte-Induced Tube Maturation in 3D Extracellular Matrices. Adv. Pharmacol 2016, 77, 241–80. [DOI] [PubMed] [Google Scholar]
- (20).Ivaska J; Heino J Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu. Rev. Cell Dev. Biol 2011, 27, 291–320. [DOI] [PubMed] [Google Scholar]
- (21).Lo HW; Hsu SC; Xia W; Cao X; Shih JY; Wei Y; Abbruzzese JL; Hortobagyi GN; Hung MC Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007, 67 (19), 9066–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Zhang Z; Dong Z; Lauxen IS; Filho MS; Nor JE Endothelial cell-secreted EGF induces epithelial to mesenchymal transition and endows head and neck cancer cells with stem-like phenotype. Cancer Res. 2014, 74 (10), 2869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Moghal N; Sternberg PW Multiple positive and negative regulators of signaling by the EGF-receptor. Curr. Opin. Cell Biol 1999, 11 (2), 190–6. [DOI] [PubMed] [Google Scholar]
- (24).Gunawardane RN; Sgroi DC; Wrobel CN; Koh E; Daley GQ; Brugge JS Novel role for PDEF in epithelial cell migration and invasion. Cancer Res. 2005, 65 (24), 11572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Morita T; Yoshizumi M; Kurihara H; Maemura K; Nagai R; Yazaki Y Shear stress increases heparin-binding epidermal growth factor-like growth factor mRNA levels in human vascular endothelial cells. Biochem. Biophys. Res. Commun 1993, 197 (1), 256–62. [DOI] [PubMed] [Google Scholar]
- (26).Sorlie T; Perou CM; Tibshirani R; Aas T; Geisler S; Johnsen H; Hastie T; Eisen MB; van de Rijn M; Jeffrey SS; Thorsen T; Quist H; Matese JC; Brown PO; Botstein D; Lonning PE; Borresen-Dale AL Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. U. S. A 2001, 98 (19), 10869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Brownfield DG; Venugopalan G; Lo A; Mori H; Tanner K; Fletcher DA; Bissell MJ Patterned collagen fibers orient branching mammary epithelium through distinct signaling modules. Curr. Biol 2013, 23 (8), 703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sokol ES; Miller DH; Breggia A; Spencer KC; Arendt LM; Gupta PB Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Res. 2016, 18 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Laschke MW; Menger MD Adipose tissue-derived microvascular fragments: natural vascularization units for regenerative medicine. Trends Biotechnol. 2015, 33 (8), 442–8. [DOI] [PubMed] [Google Scholar]
- (30).Hensley CT; Faubert B; Yuan Q; Lev-Cohain N; Jin E; Kim J; Jiang L; Ko B; Skelton R; Loudat L; Wodzak M; Klimko C; McMillan E; Butt Y; Ni M; Oliver D; Torrealba J; Malloy CR; Kernstine K; Lenkinski RE; DeBerardinis RJ Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164 (4), 681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1: MCF10A-green_HUVEC-red.mpg shows MCF10A breast epithelial cell spheroids (green) cultured on a HUVEC layer (red) for 24 h (MPG).
Movie 2: MCF10A-NeuN-red_HUVEC-green.mpg shows MCF10A-NeuN breast epithelial cell spheroids (red) cultured on a HUVEC layer (green) for 24 h (MPG).