Abstract
Cold-water corals are threatened by global warming, especially in the Mediterranean Sea where they live close to their upper known thermal limit (i.e. 13°C), yet their response to rising temperatures is not well known. Here, temperature effects on Lophelia pertusa and Madrepora oculata holobionts (i.e. the host and its associated microbiome) were investigated. We found that at warmer seawater temperature (+2°C), L. pertusa showed a modification of its microbiome prior to a change in behaviour, leading to lower energy reserves and skeletal growth, whereas M. oculata was more resilient. At extreme temperature (+4°C), both species quickly lost their specific bacterial signature followed by lower physiological activity prior to death. In addition, our results showing the holobionts' negative response to colder temperatures (−3°C), suggest that Mediterranean corals live close to their thermal optimum. The species-specific response to temperature change highlights that global warming may affect dramatically the main deep-sea reef-builders, which would alter the associated biodiversity and related ecosystem services.
Keywords: Lophelia pertusa, Madrepora oculata, global change, bacteria, metabolic activity, colony growth rates
1. Introduction
Human activities have increased the atmospheric CO2 concentration by up to 40% [1], leading to an increase in sea temperatures [2]. The deep sea is also impacted as the effect of climate change has been observed down to several hundreds to thousands metres depth [3,4]. The Mediterranean Sea, an almost land-locked sea, is particularly exposed to the effects of global warming [5], and deep-water temperatures may increase by 1.5°C by the end of the century [2,6]. Climate change will thus impact deep-seafloor ecosystems and may well influence the distribution of suitable habitats for a number of species in the deep ocean [7,8].
Among deep-sea habitats, the reefs formed by cold-water corals (CWCs) provide niches and nursery grounds for a variety of species, including commercial species [7]. Accordingly, these deep-sea engineers of high ecological and economical values require specific conservation strategies. Unfortunately, CWC reefs are threatened by direct anthropogenic activities, and the increase of CO2 concentrations that acidifies and warms up seawater. The impacts of ocean acidification and warming on coral physiology, and particularly for calcification, have been studied recently [9–11]. The integrative effects of climate change at different biological scales in the coral holobiont are, however, poorly known. Additionally, tolerance to temperature changes could differ between species, as suggested by the opposite responses in calcification rates observed for Dendrophyllia cornigera and the solitary cup coral Desmophyllum dianthus [10,12].
The main deep-sea engineers Lophelia pertusa (recently synonymized to Desmophyllum pertusum [13]) and Madrepora oculata exhibit different ecological strategies in terms of growth, nutrition, reproduction and bacterial community composition [14–16], suggesting different responses to environmental changes. The impact of climate change on the two species is still debated, as fossils and recent analyses of corals from the Mediterranean Sea and the Atlantic Ocean have suggested a higher temperature tolerance for M. oculata compared to L. pertusa [17–20], while short-term aquaria experiments have described lower thermal acclimation potential for calcification and respiration [21].
Compared to their shallow-water counterparts, the study of global warming effects on reef-building CWCs is still in its infancy. For tropical corals, higher seawater temperatures impact colony calcification and induce expulsion of symbiotic zooxanthella (so-called coral bleaching), with the emergence of diseases leading to significant mortality rates [22]. More recently, studies on tropical octocorals and scleractinians at the holobiont level revealed the role of the microbiome for coral resilience to changing environmental conditions [23–25]. A novel holobiont approach would be useful for deep-sea corals and might provide clues to determine their resilience to future changes.
The aim of this study was to investigate the effects of seawater temperature changes on the main deep engineer coral species, L. pertusa and M. oculata, from the Mediterranean Sea, where they live close to their upper known thermal limit (i.e. 13°C). We tested whether cooler (10°C) or warmer seawater temperatures (15°C and 17°C) had an effect on the coral-associated microbial communities, behaviour (prey capture rates and polyp activity), energy budget (total organic matter (TOM) and lipid, protein and carbohydrate tissue contents), skeletal growth rates and polyp survival. We conducted investigations on CWCs at the holobiont level for the first time, to provide a more complete view of the physiological pathways that could be affected by global warming, at short (one week) and longer (two and six months) timescales. A species-specific response to seawater temperature changes could in turn change the composition of CWC communities in the deep sea.
2. Material and methods
(a) . Coral sampling
Specimens of L. pertusa and M. oculata were collected at 540 m depth from the Lacaze-Duthiers canyon in the Gulf of Lion, in the northwestern Mediterranean Sea (42°32′72″ N, 03°25′28″ E) using the remotely operated vehicle (ROV) Super Achille from the R/V Janus II ship (COMEX Company). Three distinct living colonies of each species were sampled within a small area (less than 70 m2) explored earlier [15,16]. Coral fragments were then transported to the sea surface using thermally insulated polypropylene boxes that maintain the ambient seawater temperature (i.e. 13°C) and transferred on board into aerated 30 l seawater tanks maintained at 13°C using a chiller. Once at the laboratory (Banyuls Oceanological Observatory), coral colonies were cut into nubbins that contained 4–25 living polyps for L. pertusa and 6–30 living polyps for M. oculata. The nubbins were glued onto cement blocks using an aquatic epoxy resin and then maintained in tanks for three months prior to the start of the experiment. This ensured a full recovery of the corals from the stress caused by the collection and cutting, and allowed acclimatization to laboratory conditions. During that time, coral nubbins were placed in a dark thermoregulated room at 13°C in aerated 80 l tanks that received continuous flow (1.5 renewal day−1) of filtered (5 µm) Mediterranean seawater pumped from 10 m below the surface. They were fed alternatively three times per week with Artemia and MarineSnow plankton diet (ratio 2 : 1, respectively).
(b) . Experimental design
Experiments were conducted in four different tanks to maintain corals at 10°C, 13°C, 15°C and 17°C (electronic supplementary material, appendix and figure S1), where 13°C represented the in situ temperature (= control). The tank at 15°C represented the temperature forecast by the IPCC [2] for the end of this century in the deep Mediterranean Sea, and 17°C simulated the extreme warmest conditions. Finally, 10°C was used to investigate if corals maintained their health conditions at colder than present Mediterranean temperatures. This temperature corresponds to the thermal conditions of North Atlantic corals that thrive at 10°C. It also allows a direct comparison with earlier studies on the response of L. pertusa and M. oculata's oxygen consumption and calcification at 10°C [11,21].
Experiments were conducted following the recommendations made in Orejas et al. [26] for CWC aquaria studies. Coral nubbins from three different colonies of each species were transferred and randomly distributed in 36 l experimental tanks. A total of 62 nubbins (32 for L. pertusa and 30 for M. oculata) were placed in each tank, with enough distance between nubbins to avoid any contact among the polyps [26]. These 62 nubbins contained overall 280 polyps of L. pertusa and 350 polyps of M. oculata. Each experimental tank was equipped with a small water pump to maintain a constant flow (3 cm s−1), and placed in a larger water bath tank to ensure stable temperature regulation. The temperature was maintained by a temperature controller (Biotherm, HOBBY, precise at 0.1°C), coupled with titanium resistances or chillers, depending on the conditions. An additional acclimation period of one month in the experimental aquaria was applied to the nubbins prior to temperature changes. Then, the experimental temperatures were linearly achieved over 7 days to avoid physiological shock following the protocol described by Naumann et al. [21]. The temperature in each tank was monitored every 10 min using an autonomous IBUTTON probe and manually taken twice a day using a digital thermometer. The pH and oxygen concentrations were measured manually every week (pH between 8 and 8.2 and oxygen concentration over 90% saturation).
During the experiment, the corals were fed three times a week alternately with freshly hatched Artemia salina nauplii (350 l−1) and with MarineSnow plankton diet (Two Little Fishies Inc., Miami Gardens, USA, 5 ml per flume), to provide a rich nutrient supply.
(c) . Skeletal growth, behaviour and energy reserves
The skeletal growth, behaviour and energy reserves were measured for each temperature condition. The skeletal growth was assessed through the polyp linear growth rate, based on the use of calcein staining [27] easily recognized under fluorescent light microscopy (electronic supplementary material, figure S2), following the protocol described in Chapron et al. [28]. Skeletal growth was also monitored with the measures of calcification rates from the buoyant techniques [29]. The detailed protocols of both polyp linear growth rate and calcification rates from the buoyant weight are provided as electronic supplementary material.
Behaviour was assessed by measuring prey capture rates and polyp activity at the start of the experiment (T0), at one week, and every month until the end of the six-month experiment. The prey capture rates for each coral species were estimated using the protocol described by Purser et al. [30] by assessing Artemia salina nauplii concentrations in the seawater before (350 l−1) and after feeding, and by normalizing to the number of polyps (electronic supplementary material). Polyp activity was inferred from video survey of tentacles movement during feeding, based on a method modified from Chapron et al. [28] (electronic supplementary material, figure S2). Briefly, we compared pictures extracted from the video and highlighted the number of changing pixels that register tentacle movements electronic supplementary material).
The energy reserves were measured as the TOM and the three main classes of compounds (lipids, proteins and carbohydrates) at the start of the experiment (T0) and at two and six months. The TOM, lipids, proteins and carbohydrates were extracted from the freeze-dried nubbins (electronic supplementary material). The TOM weight represents the difference between the weight before and after combustion at 450°C. Lipids were quantified following the colorimetric assay developed by Barnes & Blackstock [31] with cholesterol as standard, proteins were determined by the Bradford method [32] with serum albumin and γ globulin as standard, and the carbohydrates were determined according to Dubois et al. [33] with glucose as standard (electronic supplementary material).
(d) . Coral bacterial communities
Coral bacterial communities for each temperature condition were assessed from three polyps (including tissues, gut and mucus) per colony at the start of the experiment (T0) and at one week, two months and six months. One litre of aquarium seawater was sampled at the same times, and filtered on 0.22 µm filter after prefiltration through a 3 µm pore-size polycarbonate filter (Millipore). Coral samples and the 0.22 µm filters were flash frozen in liquid nitrogen and then stored at −80°C until nucleic acid extractions. For DNA extraction, individual polyps were crushed using a sterile hammer and tissues were homogenized in tubes containing a garnet matrix (Lysing Matrix A without the large ball, MP, Biomedical, Santa Ana, CA, United States), and lysed mechanically using a FastPrep Instrument (MP, Biomedical) (adapted from Galand et al. [34]). DNA extraction was performed using the Maxwell Blood DNA Purification Kit LEV and the Maxwell 16 MDx Instrument (Promega, Madison, WI, United States) following the manufacturer's instructions. The same protocol was applied for the seawater bacteria community after cutting the filters in small fragments before mechanical lysis.
For the sequencing, the V1–V3 region of the bacterial 16S rRNA genes were amplified using the primers 27F AGRGTTTGATCMTGGCTCAG and 519R GTNTTACNGCGGCKGCTG with a single step and 28 cycles of polymerase chain reaction (PCR) using the HotStarTaq Plus Master Mix Kit (Qiagen, Valencia, CA, United States). Amplicon fragments were PCR-amplified using the high-fidelity Phusion polymerase under conditions of 30 s at 98°C, 16 cycles of 98°C for 10 s, 60°C for 30 s, 72°C for 80 s and final extension for 5 m at 72°C. Following the PCR, all the amplicon products from the different samples were mixed in equal concentrations and purified using Agencourt Ampure beads (Agencourt Bioscience Corporation, MA, United States). The DNA library was prepared using the purified PCR products following the Illumina TruSeq DNA library preparation protocol. All the samples were sequenced on the same Miseq Illumina sequencer run using Miseq reagent kit V3 (Illumina) producing 2 × 300-bp long reads. PCR and sequencing were conducted in a commercial laboratory (Integrated Microbiome Resource, Halifax, Canada). All sequences were deposited in GenBank under SRA accession number PRJNA648865.
Sequence analysis was performed with DADA2 in ‘R’ to analyse and correct Illumina-sequenced amplicon errors [35]. We applied the standard pipeline with the following parameters: trimLeft = 21, truncLen = c(290, 250), maxN = 0, maxEE = c(5,5), truncQ = 2. The sequences were filtered, dereplicated, and chimeras removed, to obtain amplicon sequence variants (ASVs). ASVs were classified against the SILVA v. 128 database [36] for taxonomic assignment. An additional BLAST [37] search was performed on the ASVs selected by SIMPER analysis from the vegan package. Samples containing less than 100 reads were removed (17 samples).
(e) . Statistical analysis
Tests for normality of variance were performed using the Shapiro–Wilk test with the R software (v. 3.4.3). The distributions were not normal for prey capture rates, polyp activity and skeletal growth rates (p < 0.05), so a multiple comparison non-parametric Kruskal–Wallis (K–W) test was used to test possible statistical differences among thermal conditions and sample times for each parameter. For the energy storages, the distribution of TOM, lipids, proteins and carbohydrates were normal allowing a multiple factors ANOVA analysis. Tukey Post hoc tests were performed to determine differences among conditions.
To enable comparison of the bacterial community compositions and diversities, the sequence data were normalized by dividing counts for each sample by the sample's size to obtain a relative abundance (average of 2500 reads per sample). Comparison of bacterial community compositions was assessed by correspondence analysis (CA) with the phyloseq package [38] in R. The Bray–Curtis index and the Shannon diversity index were computed between and within samples with the vegan package [39]. The bacterial diversity data were compared among coral species, temperature and time of exposure using analysis of similarities (ANOSIM). ANOSIM is a non-parametric test that uses a dissimilarity matrix as input to determine if there are significant differences in the microbial community composition between groups of samples. A simper test was applied with the plyr package [40] to identify the ASVs that characterized the different samples.
3. Results
(a) . Polyp survival
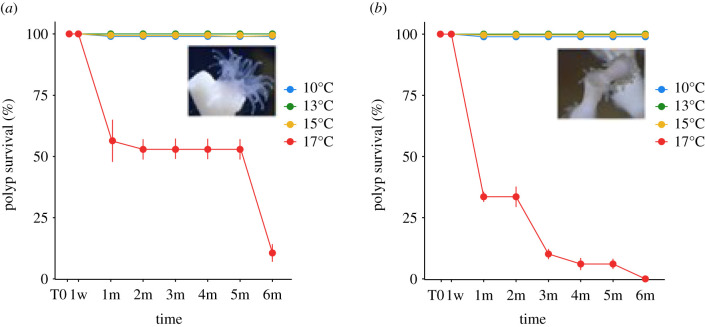
There was no polyp mortality for either of the two species at 10°C, 13°C and 15°C during the whole experiment. At 17°C, polyp survival of L. pertusa decreased to 54 ± 14% at one month, then to 20 ± 8% at six months. For M. oculata, polyp survival decreased gradually with 30 ± 2% at one month to 0% at six months (figure 1).
Figure 1.
Polyp survival rate (%) for (a) L. pertusa and (b) M. oculata at 10°C, 13°C, 15°C and 17°C during six months of the experiment. T0: start of the experiment, w: week, m: month. Mean values (from three colony replicates per time point) and standard deviations are presented. (Online version in colour.)
(b) . Skeletal growth
Overall, L. pertusa had a significantly higher polyp linear growth rate than M. oculata (average of 2.5 ± 2.8 mm yr−1 and 0.6 ± 0.4 mm yr−1 respectively, table 1, electronic supplementary material, figure S3, K–W, n = 295, p < 0.01). Lophelia pertusa apical polyps, namely those produced at branch summits and which drive the linear extension of the colony (electronic supplementary material, appendix and figure S4), grew faster than their subapical polyps (average of 3.7 ± 3.5 mm yr−1 and 1.4 ± 1.2 mm yr−1, respectively, table 1, electronic supplementary material, figure S3, K–W, n = 87, p = 0.001). Lophelia pertusa polyp linear growth rate was significantly lower at 10°C compared to 13°C, when considering both apical and subapical polyps (K–W, n = 87, p = 0.02). When considering only apical polyps, the polyp linear growth rate was statistically lower at both 10°C (K–W, n = 44, p = 0.02) and 15°C (K–W, n = 44, p = 0.04), compared to 13°C. For M. oculata, no differences were observed among temperatures (K–W, n = 208, p > 0.05) and polyp position (K–W, n = 208, p > 0.05). The 17°C data could not be used due to the high mortality observed already after two months leading to the absence of biological material at six months for M. oculata. For L. pertusa, the few remaining living polyps were used to assess coral bacterial community composition.
Table 1.
Average polyp linear growth rates ± s.d. (mm yr−1) of L. pertusa and M. oculata for all, apical and subapical polyps, under different temperature conditions after six months of incubation. The number in brackets represent the number of replicates.
| all polyps (mm yr−1) | apical polyps (mm yr−1) | subapical polyps (mm yr−1) | ||
|---|---|---|---|---|
| Lophelia pertusa | all | 2.5 ± 2.8 (87) | 3.7 ± 3.5 (44) | 1.4 ± 1.2 (43) |
| 10°C | 1.6 ± 2.3 (31) | 2.7 ± 2.9 (14) | 0.7 ± 0.9 (17) | |
| 13°C | 3.6 ± 3.3 (26) | 4.9 ± 4.1 (13) | 2.3 ± 1.3 (13) | |
| 15°C | 2.6 ± 2.7 (30) | 3.5 ± 3.3 (17) | 1.4 ± 0.7 (13) | |
| Madrepora oculata | all | 0.6 ± 0.4 (208) | 0.5 ± 0.3 (58) | 0.6 ± 0.4 (150) |
| 10°C | 0.6 ± 0.4 (62) | 0.6 ± 0.3 (17) | 0.7 ± 0.4 (45) | |
| 13°C | 0.6 ± 0.3 (75) | 0.5 ± 0.2 (19) | 0.7 ± 0.4 (56) | |
| 15°C | 0.5 ± 0.4 (71) | 0.5 ± 0.4 (22) | 0.6 ± 0.4 (49) |
Based on the buoyant weight data, overall, L. pertusa had a significantly higher calcification rate than M. oculata (average of 0.023 and 0.015 G % day−1, respectively, K–W, p < 0.05, n = 116, electronic supplementary material, appendix and figure S5). Within both species, there were no significant differences in calcification rates among temperatures.
(c) . Behaviour
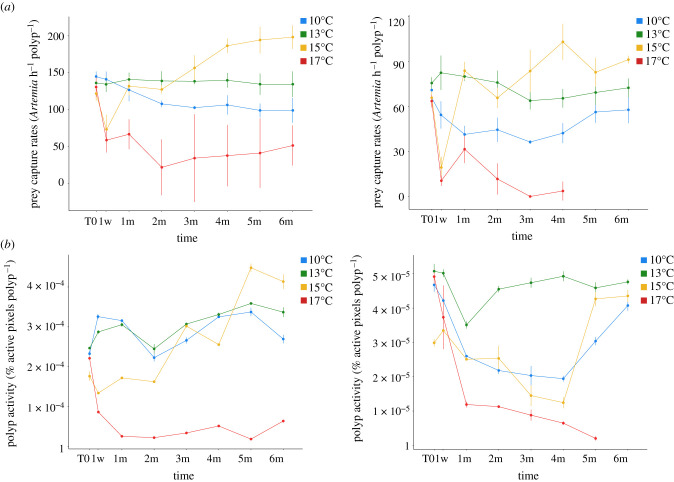
Lophelia pertusa had significantly higher prey capture rates and polyp activity compared to M. oculata (figure 2, K–W, p < 0.05, figure 3). Under control temperature condition (13°C), these parameters did not change during the whole experiment for L. pertusa and M. oculata (K–W, p < 0.05).
Figure 2.
Average prey capture rate (a) and average polyp activity (b) of L. pertusa (left) and M. oculata (right) during the first hour of feeding for six months of the experiment at 10°C, 13°C, 15°C and 17°C. Mean values (from three replicates per time point) and standard deviations are presented. (Online version in colour.)
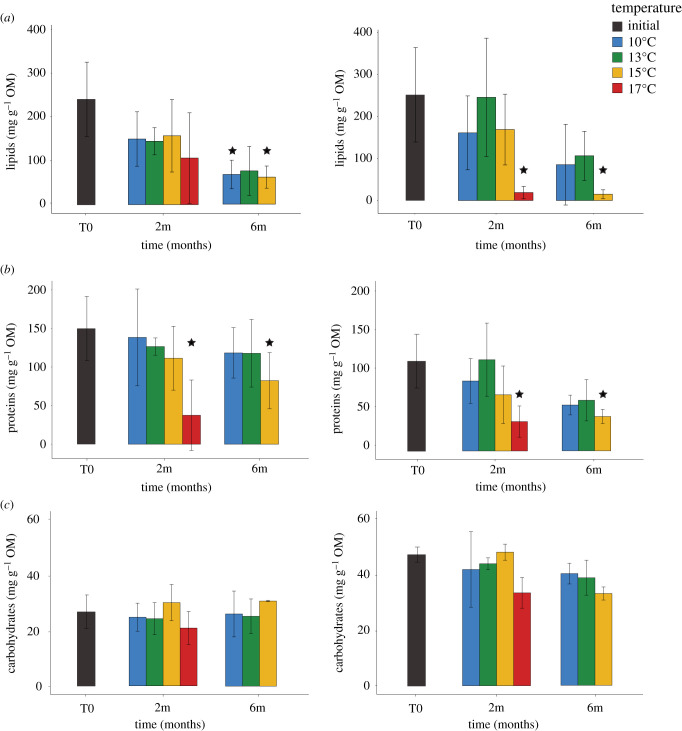
Figure 3.
Average concentrations of (a) lipids, (b) proteins and (c) carbohydrates at the start of the experiment (T0), and at two and six months at 10°C, 13°C, 15°C and 17°C for L. pertusa (left) and M. oculata (right). Mean values (from three replicates per time point) and standard deviations are presented. The stars represent the significant differences between values from temperature exposures and the values from the start of the experiment (T0, ANOVA, p < 0.05). (Online version in colour.)
At 10°C, significantly lower values compared to control were observed at six months for L. pertusa (K–W, p < 0.05, figure 2). For M. oculata, prey capture rates and polyp activity were significantly lower than control (K–W, p < 0.05) during the first four months then the values increased to reach control condition levels.
At 15°C, the prey capture rates were at their lowest at one week for both species, and then increased gradually to be significantly higher than the control after three months (figure 2; K–W, p < 0.05). The same pattern was observed for L. pertusa's polyp activity, in contrast to M. oculata's that decreased gradually the first four months, and then increased to reach control values after five months (K–W, p > 0.05).
At 17°C, both species showed significantly lower prey capture rates and polyp activity than control from one week to the end of the experiment (figure 2; K–W, p > 0.05).
(d) . Energy storage
The TOM content did not differ between species during the whole experiment (electronic supplementary material, appendix and figure S5, ANOVA p > 0.05). No difference was observed at 10°C compared to control values. TOM contents were significantly lower at six months for 15°C (electronic supplementary material, appendix and figure S5, ANOVA p < 0.05), and lower at two months for 17°C (electronic supplementary material, appendix and figure S5, ANOVA p < 0.05).
At the start of the experiment, both species had higher concentration of lipids and then proteins, compared to carbohydrates (figure 3). At 10°C, the lipid concentrations were significantly lower than control at six months for L. pertusa but not for M. oculata. Both species exhibited the same trends for warmer temperatures (figure 3a,b), with lower lipid and protein concentrations compared to control at six months for 15°C, and at two months for 17°C (with the exception of lipids from L. pertusa due to a high standard deviation value). For both species, lipids were the dominant component of the TOM at the beginning of the experiment, then proteins became the most abundant component at the end for all temperatures for L. pertusa, and at 15°C and 17°C for M. oculata (figure 3a,b). For carbohydrates, there were no differences among temperatures for either species (figure 3c).
(e) . Coral bacterial communities
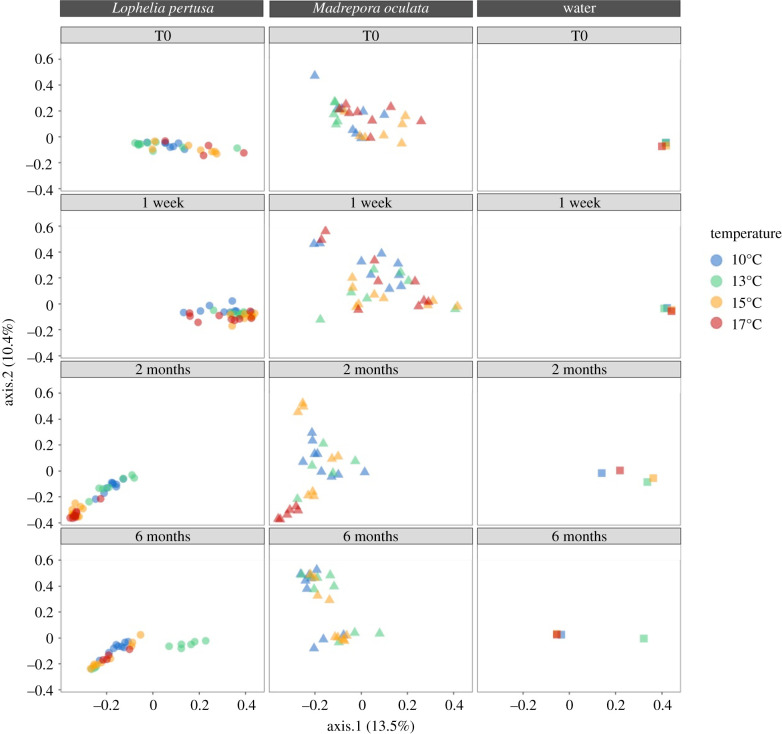
The seawater bacterial communities were similar between all tanks at the start of the experiment (T0), and differed from the coral bacterial composition (figure 4; electronic supplementary material, appendix and table S1). At the start of the experiment (T0), L. pertusa and M. oculata had different bacterial communities (figure 4; electronic supplementary material, appendix and table S1, ANOSIM, p < 0.01).
Figure 4.
Correspondence analysis of bacterial community composition of L. pertusa, M. oculata and seawater at the start of the experiment (T0), at one week, two months and six months at 10°C, 13°C, 15°C and 17°C. Bacterial communities are described based on 16S rRNA gene. The same scale is used for all ordination axes. (Online version in colour.)
Under control conditions (13°C), the coral bacterial community compositions for both species L. pertusa and M. oculata did not change significantly through time (figure 4; electronic supplementary material, appendix, ANOSIM, p > 0.01).
Under experimental conditions, at one week, no change in bacterial community composition was observed in both L. pertusa and M. oculata (electronic supplementary material, appendix and table S2, ANOSIM p > 0.05). At two months, the microbiome of L. pertusa at 15°C and 17°C became similar to each other, separated from 10°C to 13°C microbiomes, and converged in their composition with the microbiome of M. oculata at 17°C (figure 4; electronic supplementary material, appendix and table S2). At six months, L. pertusa's microbiome at 10°C, in turn, became similar to the microbiome at 15°C and 17°C. By contrast in M. oculata, the microbiomes at 10°C, 13°C and 15°C were similar to each other and did not change with time (figure 4; electronic supplementary material, appendix and table S2). No data were available for M. oculata after six months at 17°C as all polyps were dead.
The bacterial communities always showed higher diversity in L. pertusa than in M. oculata (Shannon diversity index, ANOVA p < 0.01). This diversity increased through time at each temperature for L. pertusa, while it remains stable for M. oculata, except at two months for 17°C, where an increase was observed (electronic supplementary material, appendix and figure S7).
At the start of the experiment and in control condition, the ASV1 (order Cellvibrionales) was the most abundant in L. pertusa (14%) and the ASV6 (Spirochaetales) was the most abundant in M. oculata (15%). Most of these ASVs were most closely related to sequences previously retrieved from shallow-water coral species (electronic supplementary material, appendix and table S3).
At 10°C, the ASV1 and ASV42 (Rhodobacterales) were the most abundant at one week in both coral species, then the ASV12 (Rhiziobiales) at two months, and the ASV49 (Rhodobacterales) at six months (electronic supplementary material, figure S8).
At 15°C and 17°C, both L. pertusa and M. oculata bacterial communities were dominated by the ASV1 and ASV24 (order Rhodobacterales) at one week. Then, they were dominated by the ASV4 (order Rhodobacterales) and ASV3 (order Acidimicrobiales) at two months, with significantly higher abundances at 17°C (electronic supplementary material, figure S8 and table S3). The ASV23 (Epsilonproteobacteria) also increased in relative abundance at 17°C for both species at two months. The ASV24 is 100% similar to sequences found in bacteria growing on living [41] or inert surfaces [42].
Most of these dominant ASVs characterizing lower or higher temperatures were not related to sequences previously found on corals (electronic supplementary material, appendix and table S3).
4. Discussion
Present Mediterranean CWCs are found at approximately 13°C, but deep-water temperatures are forecasted to increase by 1.5°C [2,6]. The absence of polyp mortality at 15°C suggests that both L. pertusa and M. oculata should tolerate the temperature expected at the end of this century. However, a 2°C increase significantly impacted L. pertusa at the holobiont level, with lower skeletal growth, energy reserves (except for carbohydrates, which are not a major component of the total energy storages in corals), altered behaviour and changes in coral microbiome. These changes appeared less pronounced for M. oculata, which may influence coral community composition in the future deep sea.
The reduced growth rate of L. pertusa at warmer temperatures may be due to an alteration of the coral metabolism as suggested by the reduction of their energy reserves at six months, even though they increased their prey capture rate through a higher polyp activity. This behavioural compensatory response to increase food intake is noteworthy and supposed to maintain vital energy levels [8,25]. It was observed earlier for L. pertusa, which maintained its skeletal growth despite an increase in temperature from 8°C to 12°C [43]. In our experiment, however, the higher prey capture rates did not appear to mitigate the increase in metabolic demand as seen by the decrease in energy reserves and skeletal growth. When lipid storage becomes depleted, proteins can increasingly be used as an energy source [44,45], as observed here with the progressive adjustment from a lipid dominated catabolism to a higher degree of protein breakdown, leading to a physiological shift since corals invest mostly in survival rather than growth [46,47]. Thus, despite the absence of mortality, future temperature (15°C) may reduce the capacity of L. pertusa to produce large reef structures.
The microbiome of L. pertusa was also affected at 15°C, with a change in microbial community composition within two months. These early changes in the microbial communities indicate that dysbiosis (change in bacterial community composition, including opportunist species) can occur rapidly with increasing temperature. This may impact the metabolic activity and energy acquisition of the host, as associated bacteria are involved in key host physiological functions such as immune response [48,49] and nutrient acquisition [50]. We observed the dominance of bacteria from the Rhodobacterales class, which could be opportunists and/or pathogens that invade the host when temperature increases. Rhodobacterales have been seen earlier growing on various submerged surfaces [42] and were linked to stress and/or coral diseases [51,52]. Their increase in abundance may thus reflect that the host, or its microbiome, are not able to control invading microorganisms.
Our dataset at the holobiont scale could indicate that 15°C is a threshold temperature for the maintenance of L. pertusa fitness. This assumption is consistent with previous observations made on L. pertusa from the Gulf of Mexico [53] suggesting that 15°C could be the upper thermal limit for this species. Our results are also in line with experiments on a closely related Mediterranean species, the solitary coral D. dianthus, which show lower calcification rates when the temperature reaches 15°C [10]. However, the stability of the bacterial communities during the first week of the experiment supports the idea that L. pertusa can tolerate short-term exposures to 15°C, as suggested through thermal tolerance curves established for specimens from the Arctic and boreal reefs [54].
Conversely, M. oculata's skeletal growth was not affected at 15°C, even though patterns in prey capture rates, polyp activity and energy reserves were similar to L. pertusa, including the change from lipid dominated to much less efficient protein-dominated metabolism. Madrepora oculata's strategy to increase food intake and use lipid reserves seems to handle the increasing metabolic demands and sustain colony growth. Interestingly, no significant change in microbial community composition was registered at 15°C, suggesting that M. oculata has a more robust microbiome than L. pertusa. The stability of M. oculata microbiome under thermal stress, although some opportunistic bacteria (e.g. Rhodobacterales) were also observed, may reflect a good health status and/or a higher resilience to environmental changes as observed for shallow-water tropical corals [23–25]. A higher temporal and geographical stability M. oculata microbiome compared to L. pertusa has already been observed in situ and in aquaria [16,34,55]. Part of the core bacterial community of CWCs could represent a food influenced microbiome [56,57], and its persistence may support the metabolic activity of the coral. Higher tolerance to warming for M. oculata than L. pertusa may explain the current distribution of these two species with the dominance of the former at shallower depths in the Mediterranean Sea, [18–20], where temperatures increases frequently to 15°C due to dense water shelf cascades [58–60].
An increase of 4°C (i.e. 17°C), rapidly led to high polyp mortality for both L. pertusa and M. oculata. Our results thus suggest that the main reef-builders in the deep sea are not resilient to larger thermal changes. Short-term experiments with L. pertusa from the Gulf of Mexico also reported low survival for such warmer temperatures [53]. The responses that we observed contrast, however, with other CWC scleractinians, such as D. cornigera, which tolerate temperatures beyond 17°C [61,62], but with alterations of physiological parameters (i.e. growth rate, calcification, respiration, total organic carbon release) at longer exposure times [63]. Interestingly, several health alerts occurred prior to death for both species with prey capture rates and feeding activity fell rapidly, and became insufficient to mitigate the metabolic demands, leading to reduced energy reserves. Both holobionts lost their original species-specific microbial communities that became similar to each other. Bacteria known to be associated with stressed or diseased corals (Rhodobacterales, Acidimicrobiales, Epsilonproteobacteria) [51,52] invaded the host rapidly (since one week), which might lead to coral dysbiosis and death. Dysbiosis appeared prior to changes in host physiology and the microbial signature could, therefore, be used as a promising sentinel of coral health [64].
Surprisingly, our holobiont approach suggests that colder temperatures (10°C) are not associated with better health, as both species acquired bacteria associated with stressed corals (e.g. Rhodobacterales) [57]. The physiological response between hosts differed, however, with a stronger impact on L. pertusa's energy reserves and skeletal growth. Previous studies on L. pertusa have reported lower calcification, respiration, ammonium and nitrogen excretion rates when the temperature decreases [11,54,61]. This suggests that even though this species tolerates a wide range of temperatures, Mediterranean specimens live close to their optimum temperature (i.e. 13°C). Further research is required to investigate if this optimum is strictly thermo-dependent or rather reflects regional adaptation processes. Our findings need nevertheless to be considered with caution as they originate from laboratory experiments in which the replicates for each colony and condition were maintained in the same tank. In addition, this study does not allow inferences regarding the dynamics of the microbiome and energy reserves between sampling times.
5. Conclusion
This is the first CWC study on thermal stress conducted at the holobiont level. We showed that warmer seawater temperatures alter the physiology and the microbiome of both L. pertusa and M. oculata. The predicted future temperature of the deep sea (15°C) strongly affected L. pertusa, while M. oculata appeared more resilient, although both species collapsed at 17°C. The cost for survival, which involves switching to different metabolic pathways and behaviour, appeared to limit L. pertusa's growth, and thus, ultimately, reef extension and habitat-forming ability for the associated biodiversity. Madrepora oculata exhibited stable microbiome and growth, which could mitigate thermal stress. However, the occurrence of dysbiosis at 15°C suggests that the persistence of the future temperature in the Mediterranean Sea may impact both species. Considering the fundamental role of the Mediterranean Sea as a refugium (e.g. during glacial times) [65] and as a source for coral dispersion in the Atlantic, changes in Mediterranean CWC community composition with rising temperatures could impact deep ecosystems over a broad geographical scale [66].
Supplementary Material
Acknowledgements
Many thanks to the crew of the Janus II (COMEX) for their assistance in fieldwork. We acknowledge the support of the Biology Platform of Imaging and Cytometry (BioPIC) for microscopic analysis, the Marine Biodiversity and Biotechnology (Bio2Mar) platform for DNA extraction and CGEP IMR laboratory for the sequencing. Thanks also to Veronique Charriere and Dr Rowan McLachlan for proofreading the article.
Data accessibility
Physiological data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.djh9w0w1h [67]. All sequences were deposited in GenBank under SRA accession no. PRJNA648865. The data are provided in the electronic supplementary material [68].
Authors' contributions
L.C.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, validation, visualization, writing—original draft, writing—review and editing; P.E.G.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, resources, supervision, writing—review and editing; A.M.P.: formal analysis, validation, writing—review and editing; E.P.: conceptualization, methodology, writing—review and editing; G.V.: formal analysis, writing—review and editing; S.R.: formal analysis, writing—review and editing; F.L.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, validation, writing—review and editing. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
The authors declare no competing interests.
Funding
This work benefited from financial support from the Rovaltain Foundation (PLAISCOOL project, coordinator F.L.) and AMOR (ANR-16-CE31-0020-02, coordinator Y. Donnadieu, Cerege) and the TelluS-INTERVIE programme from CNRS-INSU (PACMAN project, coordinator F.L.). Coral samplings were done as part of the experimentation programme of the Chair ‘Biodiversity, extreme marine environment and global change’ supported by Foundation TOTAL, UPMC and CNRS, (coordinator N. Le Bris, LECOB). L.C.'s PhD grant was funded by the French Ministry of Higher Education, Research and Innovation through the Doctoral School ‘Sciences de l'Environnement d'Ile de France—ED129’.
References
- 1.Zeebe RE, Zachos JC, Caldeira K, Tyrrell T. 2008. OCEANS: carbon emissions and acidification. Science 321, 51-52. ( 10.1126/science.1159124) [DOI] [PubMed] [Google Scholar]
- 2.IPCC. 2013. Climate change 2014: synthesis report. In Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change (eds Core Writing Team, Pachauri RK, Meyer LA), pp. 151. Geneva, Switzerland: IPCC. [Google Scholar]
- 3.Barnett TP. 2005. Penetration of human-induced warming into the world's oceans. Science 309, 284-287. ( 10.1126/science.1112418) [DOI] [PubMed] [Google Scholar]
- 4.Wijffels S, Roemmich D, Monselesan D, Church J, Gilson J. 2016. Ocean temperatures chronicle the ongoing warming of Earth. Nat. Clim. Change 6, 116-118. ( 10.1038/nclimate2924) [DOI] [Google Scholar]
- 5.Calvo E, Simó R, Coma R, Ribes M, Pascual J, Sabatés A, Gili JM, Pelejero C. 2011. Effects of climate change on Mediterranean marine ecosystems: the case of the Catalan Sea. Clim. Res. 50, 1-29. ( 10.3354/cr01040) [DOI] [Google Scholar]
- 6.Somot S, Sevault F, Déqué M. 2006. Transient climate change scenario simulation of the Mediterranean Sea for the twenty-first century using a high-resolution ocean circulation model. Clim. Dyn. 27, 851-879. ( 10.1007/s00382-006-0167-z) [DOI] [Google Scholar]
- 7.Roberts JM. 2006. Reefs of the deep: the biology and geology of cold-water coral ecosystems. Science 312, 543-547. ( 10.1126/science.1119861) [DOI] [PubMed] [Google Scholar]
- 8.Morato T, et al. 2020. Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Glob. Change Biol. 26, 2181-2202. ( 10.1111/gcb.14996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodolfo-Metalpa R, et al. 2015. Calcification is not the Achilles’ heel of cold-water corals in an acidifying ocean. Glob. Change Biol. 21, 2238-2248. ( 10.1111/gcb.12867) [DOI] [PubMed] [Google Scholar]
- 10.Gori A, Ferrier-Pagès C, Hennige SJ, Murray F, Rottier C, Wicks LC, Roberts JM. 2016. Physiological response of the cold-water coral Desmophyllum dianthus to thermal stress and ocean acidification. PeerJ 4, e1606. ( 10.7717/peerj.1606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodds LA, Roberts JM, Taylor AC, Marubini F. 2007. Metabolic tolerance of the cold-water coral Lophelia pertusa (Scleractinia) to temperature and dissolved oxygen change. J. Exp. Mar. Biol. Ecol. 349, 205-214. ( 10.1016/j.jembe.2007.05.013) [DOI] [Google Scholar]
- 12.Gori A, Grover R, Orejas C, Sikorski S, Ferrier-Pagès C. 2014. Uptake of dissolved free amino acids by four cold-water coral species from the Mediterranean Sea. Deep Sea Res. II: Top. Stud. Oceanogr. 99, 42-50. ( 10.1016/j.dsr2.2013.06.007) [DOI] [Google Scholar]
- 13.Addamo AM, Vertino A, Stolarski J, García-Jiménez R, Taviani M, Machordom A. 2016. Merging scleractinian genera: the overwhelming genetic similarity between solitary Desmophyllum and colonial Lophelia. BMC Evol. Biol. 16, 108. ( 10.1186/s12862-016-0654-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waller RG, Tyler PA. 2005. The reproductive biology of two deep-water, reef-building scleractinians from the NE Atlantic Ocean. Coral Reefs 24, 514-522. ( 10.1007/s00338-005-0501-7) [DOI] [Google Scholar]
- 15.Lartaud F, et al. 2014. Temporal changes in the growth of two Mediterranean cold-water coral species, in situ and in aquaria. Deep Sea Res. II: Top. Stud. Oceanogr. 99, 64-70. ( 10.1016/j.dsr2.2013.06.024) [DOI] [Google Scholar]
- 16.Anne-L M, Lartaud F, Arnaud-Haond S, Kalenitchenko D, Bessalam M, Le Bris N, Galand PE. 2016. Patterns of bacteria–host associations suggest different ecological strategies between two reef building cold-water coral species. Deep Sea Res. I: Oceanogr. Res. Pap. 114, 12-22. ( 10.1016/j.dsr.2016.04.013) [DOI] [Google Scholar]
- 17.Wienberg C, Hebbeln D, Fink HG, Mienis F, Dorschel B, Vertino A, Correa ML, Freiwald A. 2009. Scleractinian cold-water corals in the Gulf of Cádiz—first clues about their spatial and temporal distribution. Deep Sea Res. I: Oceanogr. Res. Pap. 56, 1873-1893. ( 10.1016/j.dsr.2009.05.016) [DOI] [Google Scholar]
- 18.Fabri M-C, Pedel L, Beuck L, Galgani F, Hebbeln D, Freiwald A. 2014. Megafauna of vulnerable marine ecosystems in French mediterranean submarine canyons: spatial distribution and anthropogenic impacts. Deep Sea Res. II: Top. Stud. Oceanogr. 104, 184-207. ( 10.1016/j.dsr2.2013.06.016) [DOI] [Google Scholar]
- 19.Keller NB, Os'kina NS. 2008. Habitat temperature ranges of azooxantellate scleractinian corals in the World Ocean. Oceanology 48, 77-84. ( 10.1134/S0001437008010098) [DOI] [Google Scholar]
- 20.Gori A, et al. 2013. Bathymetrical distribution and size structure of cold-water coral populations in the Cap de Creus and Lacaze-Duthiers canyons (northwestern Mediterranean). Biogeosciences 10, 2049-2060. ( 10.5194/bg-10-2049-2013) [DOI] [Google Scholar]
- 21.Naumann MS, Orejas C, Ferrier-Pagès C. 2014. Species-specific physiological response by the cold-water corals Lophelia pertusa and Madrepora oculata to variations within their natural temperature range. Deep Sea Res. II: Top. Stud. Oceanogr. 99, 36-41. ( 10.1016/j.dsr2.2013.05.025) [DOI] [Google Scholar]
- 22.Sully S, Burkepile DE, Donovan MK, Hodgson G, van Woesik R. 2019. A global analysis of coral bleaching over the past two decades. Nat. Commun. 10, 1264. ( 10.1038/s41467-019-09238-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ziegler M, Seneca FO, Yum LK, Palumbi SR, Voolstra CR. 2017. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 8, 14213. ( 10.1038/ncomms14213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCauley M, Jackson CR, Goulet TL. 2020. Microbiomes of Caribbean octocorals vary over time but are resistant to environmental change. Front. Microbiol. 11, 1272. ( 10.3389/fmicb.2020.01272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maher RL, et al. 2020. Coral microbiomes demonstrate flexibility and resilience through a reduction in community diversity following a thermal stress event. Front. Ecol. Evol. 8, 555698. ( 10.3389/fevo.2020.555698) [DOI] [Google Scholar]
- 26.Orejas C, et al. 2019. Cold-water coral in aquaria: advances and challenges. A focus on the Mediterranean. In Mediterranean cold-water corals: past, present and future. Coral reefs of the World, vol. 9 (eds C Orejas, C Jiménez), pp. 435–471. Cham, Switzerland: Springer. [Google Scholar]
- 27.Lartaud F, Pareige S, de Rafelis M, Feuillassier L, Bideau M, Peru E, Romans P, Alcala F, Le Bris N. 2013. A new approach for assessing cold-water coral growth in situ using fluorescent calcein staining. Aquat. Living Resour. 26, 187-196. ( 10.1051/alr/2012029) [DOI] [Google Scholar]
- 28.Chapron L, et al. 2018. Macro- and microplastics affect cold-water corals growth, feeding and behaviour. Sci. Rep. 8, 15299. ( 10.1038/s41598-018-33683-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jokiel P, Maragos JE, Franzisket L. 1978. Coral growth: buoyant weight technique. In Coral reefs: research methods (eds Stoddart DR, Johannes RE), pp. 529-541. Paris, France: UNESCO. [Google Scholar]
- 30.Purser A, Larsson AI, Thomsen L, van Oevelen D. 2010. The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. J. Exp. Mar. Biol. Ecol. 395, 55-62. ( 10.1016/j.jembe.2010.08.013) [DOI] [Google Scholar]
- 31.Barnes H, Blackstock J. 1973. Estimation of lipids in marine animals and tissues: detailed investigations of the sulphophosphovanillin method for ‘total’ lipids. J. Exp. Mar. Biol. Ecol. 12, 103-118. ( 10.1016/0022-0981(73)90040-3) [DOI] [Google Scholar]
- 32.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248-254. ( 10.1016/0003-2697(76)90527-3) [DOI] [PubMed] [Google Scholar]
- 33.DuBois C, Gilles K, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350-356. ( 10.1021/ac60111a017) [DOI] [Google Scholar]
- 34.Galand PE, Chapron L, Meistertzheim A-L, Peru E, Lartaud F. 2018. The effect of captivity on the dynamics of active bacterial communities differs between two deep-sea coral species. Front. Microbiol. 9, 2565. ( 10.3389/fmicb.2018.02565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581-583. ( 10.1038/nmeth.3869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2012. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590-D596. ( 10.1093/nar/gks1219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215, 403-410. ( 10.1016/S0022-2836(05)80360-2) [DOI] [PubMed] [Google Scholar]
- 38.McMurdie PJ, Holmes S. 2013. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217. ( 10.1371/journal.pone.0061217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oksanen J, et al. 2014. Vegan: Community Ecology Package. R Package Version 2.2-0. See http://CRAN.Rproject.org/package=vegan.
- 40.Wickham H. 2011. The split–apply–combine strategy for data analysis. J. Stat. Softw. 40, 1-29. ( 10.18637/jss.v040.i01) [DOI] [Google Scholar]
- 41.Balcázar JL, Lee NM, Pintado J, Planas M. 2010. Phylogenetic characterization and in situ detection of bacterial communities associated with seahorses (Hippocampus guttulatus) in captivity. Syst. Appl. Microbiol. 33, 71-77. ( 10.1016/j.syapm.2009.11.005) [DOI] [PubMed] [Google Scholar]
- 42.Dang H, Li T, Chen M, Huang G. 2008. Cross-ocean distribution of Rhodobacterales bacteria as primary surface colonizers in temperate coastal marine waters. Appl. Environ. Microbio.l 74, 52-60. ( 10.1128/AEM.01400-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Büscher JV, Form AU, Riebesell U. 2017. Interactive effects of ocean acidification and warming on growth, fitness and survival of the cold-water coral Lophelia pertusa under different food availabilities. Front. Mar. Sci. 4, 101. ( 10.3389/fmars.2017.00101) [DOI] [Google Scholar]
- 44.Lesser MP. 2013. Using energetic budgets to assess the effects of environmental stress on corals: are we measuring the right things? Coral Reefs 32, 25-33. ( 10.1007/s00338-012-0993-x) [DOI] [Google Scholar]
- 45.Skottene E, Tarrant AM, Olsen AJ, Altin D, Østensen M-A, Hansen BH, Choquet M, Jenssen BM, Olsen RE. 2019. The β-oxidation pathway is downregulated during diapause termination in Calanus copepods. Sci. Rep. 9, 16686. ( 10.1038/s41598-019-53032-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nordemar I, Nyström M, Dizon R. 2003. Effects of elevated seawater temperature and nitrate enrichment on the branching coral Porites cylindrica in the absence of particulate food. Mar. Biol. 142, 669-677. ( 10.1007/s00227-002-0989-0) [DOI] [Google Scholar]
- 47.Scanes E, Kutti T, Fang JKH, Johnston EL, Ross PM, Bannister RJ. 2018. Mine waste and acute warming induce energetic stress in the deep-sea sponge Geodia atlantica and coral Primnoa resedeaformis; results from a mesocosm study. Front. Mar. Sci. 5, 129. ( 10.3389/fmars.2018.00129) [DOI] [Google Scholar]
- 48.Reshef L, Koren O, Loya Y, Zilber-Rosenberg I, Rosenberg E. 2006. The coral probiotic hypothesis. Environ. Microbiol. 8, 2068-2073. ( 10.1111/j.1462-2920.2006.01148.x) [DOI] [PubMed] [Google Scholar]
- 49.Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. 2007. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 5, 355-362. ( 10.1038/nrmicro1635) [DOI] [PubMed] [Google Scholar]
- 50.Krediet CJ, Ritchie KB, Paul VJ, Teplitski M. 2013. Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proc. R. Soc. B 280, 20122328. ( 10.1098/rspb.2012.2328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sekar R, Mills DK, Remily ER, Voss JD, Richardson LL. 2006. Microbial communities in the surface mucopolysaccharide layer and the black band microbial mat of black band-diseased Siderastrea siderea. Appl. Environ. Microbiol. 72, 5963-5973. ( 10.1128/AEM.00843-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meron D, Rodolfo-Metalpa R, Cunning R, Baker AC, Fine M, Banin E. 2012. Changes in coral microbial communities in response to a natural pH gradient. ISME J. 6, 1775-1785. ( 10.1038/ismej.2012.19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brooke S, Ross SW, Bane JM, Seim HE, Young CM. 2013. Temperature tolerance of the deep-sea coral Lophelia pertusa from the southeastern United States. Deep Sea Res. II: Top. Stud. Oceanogr. 92, 240-248. ( 10.1016/j.dsr2.2012.12.001) [DOI] [Google Scholar]
- 54.Dorey N, Gjelsvik Ø, Kutti T, Büscher JV. 2020. Broad thermal tolerance in the cold-water coral Lophelia pertusa from Arctic and boreal reefs. Front. Physiol. 10, 1636. ( 10.3389/fphys.2019.01636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chapron L, Lartaud F, Le Bris N, Peru E, Galand PE. 2020. Local variability in microbiome composition and growth suggests habitat preferences for two reef-building cold-water coral species. Front. Microbiol. 11, 275. ( 10.3389/fmicb.2020.00275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galand PE, Remize M, Meistertzheim A, Pruski AM, Peru E, Suhrhoff TJ, Le Bris N, Vétion G, Lartaud F. 2020. Diet shapes cold-water corals bacterial communities. Environ. Microbiol. 22, 354-368. ( 10.1111/1462-2920.14852) [DOI] [PubMed] [Google Scholar]
- 57.Neulinger SC, Jarnegren J, Ludvigsen M, Lochte K, Dullo W-C. 2008. Phenotype-specific bacterial communities in the cold-water coral Lophelia pertusa (Scleractinia) and their implications for the coral's nutrition, health, and distribution. Appl. Environ. Microbiol. 74, 7272-7285. ( 10.1128/AEM.01777-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonnin J, et al. 2008. Comparison of horizontal and downward particle fluxes across canyons of the Gulf of Lions (NW Mediterranean): meteorological and hydrodynamical forcing. Cont. Shelf Res. 28, 1957-1970. ( 10.1016/j.csr.2008.06.004) [DOI] [Google Scholar]
- 59.Ogston AS, Drexler TM, Puig P. 2008. Sediment delivery, resuspension, and transport in two contrasting canyon environments in the southwest Gulf of Lions. Cont. Shelf Res. 28, 2000-2016. ( 10.1016/j.csr.2008.02.012) [DOI] [Google Scholar]
- 60.Palanques A, de Madron XD, Puig P, Fabres J, Guillén J, Calafat A, Canals M, Heussner S, Bonnin J. 2006. Suspended sediment fluxes and transport processes in the Gulf of Lions submarine canyons. The role of storms and dense water cascading. Mar. Geol. 234, 43-61. ( 10.1016/j.margeo.2006.09.002) [DOI] [Google Scholar]
- 61.Naumann MS, Orejas C, Ferrier-Pages C. 2013. High thermal tolerance of two Mediterranean cold-water coral species maintained in aquaria. Coral Reefs 32, 749-754. ( 10.1007/s00338-013-1011-7) [DOI] [Google Scholar]
- 62.Castellan G, Angeletti L, Taviani M, Montagna P. 2019. The yellow coral Dendrophyllia cornigera in a warming ocean. Front. Mar. Sci. 6, 692. ( 10.3389/fmars.2019.00692) [DOI] [Google Scholar]
- 63.Reynaud S, Orejas C, Campagno A, Rottier C, Jimenez C, Ferrier-Pagès C. 2021. Dendrophylliidae cold-water corals in a warm ocean: the effect of exposure duration on their physiological response. Deep Sea Res. II: Top. Stud. Oceanogr. 193, 104962. ( 10.1016/j.dsr2.2021.104962) [DOI] [Google Scholar]
- 64.Glasl B, Bourne DG, Frade PR, Thomas T, Schaffelke B, Webster NS. 2019. Microbial indicators of environmental perturbations in coral reef ecosystems. Microbiome 7, 94. ( 10.1186/s40168-019-0705-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boavida J, et al. 2019. Out of the mediterranean? Post-glacial colonization pathways varied among cold-water coral species. J. Biogeogr. 46, 915-931. ( 10.1111/jbi.13570) [DOI] [Google Scholar]
- 66.Kubicek A, Breckling B, Hoegh-Guldberg O, Reuter H. 2019. Climate change drives trait-shifts in coral reef communities. Sci. Rep. 9, 3721. ( 10.1038/s41598-019-38962-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chapron L, Galand PE, Pruski AM, Peru E, Vétion G, Robin S, Lartaud F. 2021. Data from: Resilience of cold water coral holobionts to thermal stress. Dryad Digital Repository. ( 10.5061/dryad.djh9w0w1h) [DOI] [PMC free article] [PubMed]
- 68.Chapron L, Galand PE, Pruski AM, Peru E, Vétion G, Robin S, Lartaud F. 2021. Resilience of cold water coral holobionts to thermal stress. Figshare. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Chapron L, Galand PE, Pruski AM, Peru E, Vétion G, Robin S, Lartaud F. 2021. Data from: Resilience of cold water coral holobionts to thermal stress. Dryad Digital Repository. ( 10.5061/dryad.djh9w0w1h) [DOI] [PMC free article] [PubMed]
- Chapron L, Galand PE, Pruski AM, Peru E, Vétion G, Robin S, Lartaud F. 2021. Resilience of cold water coral holobionts to thermal stress. Figshare. [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Physiological data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.djh9w0w1h [67]. All sequences were deposited in GenBank under SRA accession no. PRJNA648865. The data are provided in the electronic supplementary material [68].