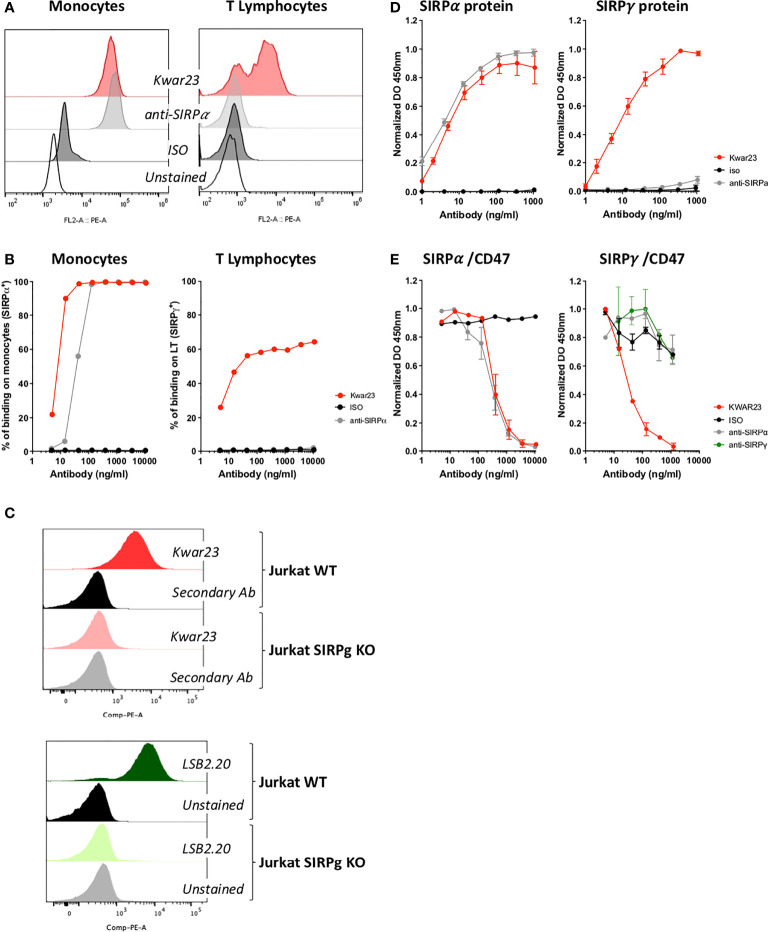
Figure 1.
The KWAR23 is a Pan SIRP mAb which impairs SIRPα and SIRPγ interactions with CD47. (A) Human monocytes and T cells were labeled with KWAR23, anti-SIRPα mAb (18D5) or an isotype control (ISO) all at 10,000 ng/ml and further analyzed by FACS. (B) The percentage of stained cells (monocytes and T cells) is presented regarding the concentration of mAb (n = 1). (C) KWAR23-binding specificity towards SIRPγ was evaluated by FACS staining on wild-type (WT) and SIRPγ−KO Jurkat cells (upper overlay). LSB2.20 (anti-SIRPγ)KWAR23 recognized staining was used as control (lower overlay). (D) Specificity of mAb used in (A) and (B) were assessed by ELISA against recombinant SIRPα and SIRPγ proteins. Binding activity is presented as normalized absorbance (DO) at 450 nm in function of concentration of mAb (ng/ml), n = 2–4 individual experiments (mean ± SEM). (E) Antagonist activity of the KWAR23, the anti-SIRPα (18D5), and the anti-SIRPγ (LSB2.20) mAbs to SIRPα/CD47 (left panel) and SIRPγ/CD47 (right panel) binding was measured by ELISA and presented as normalized absorbance (DO) at 450 nm in function of mAb added (ng/ml). n = 3 individual experiments (mean ± SEM).