Eosinophilic esophagitis (EoE) is a chronic condition that appears to be increasing in prevalence worldwide. In the 2012 United States Medicaid population, we recently reported that EoE is less prevalent in rural areas of the United States, as well as among those living in higher poverty neighborhoods.(1) While it is possible that these findings could be related to unique urban exposures such as pollution, it may also be that the apparent protective effect of rural residence is instead related to under-diagnosis of EoE. In particular, there may be lack of access to pediatric subspecialty care — there are few pediatric gastroenterologists in rural areas and most of these subspecialty providers are located in urban areas.(2) The objective of this study was to determine whether rural status remains protective for EoE diagnosis when adjusting for distance to pediatric gastroenterology provider.
This was a cross-sectional study of individuals aged 0–17 years enrolled in Medicaid for the entirety of 2012. As previously described, the Medicaid data were collected and aggregated on the state level and then processed by the Centers for Medicare and Medicaid into the Medicaid Analytic Extract (MAX), with individuals grouped by zip-code tabulation area (ZCTA).(1) The ZCTA data was then linked to the 2013 NCHS Urban-Rural Classification scheme for Counties, based on the containing county, and the 2011 American Community Survey. EoE was defined as having at least one billed event (hospitalization, Emergency Room visit, or outpatient encounter) with an International Classification of Diseases, Ninth Revision (ICD-9) code for EoE (530.13) during the 12-month period.
Geographic information on pediatric gastroenterology providers in 2012 was obtained from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) database (n=2,147). This provider list was restricted to individuals with an MD or DO degree, without emeritus status, practicing in the United States with an available zip code (n=1,496). The spatial location of these providers was then assigned to the corresponding ZCTA centroid. To estimate distance to a provider within an individual’s state, we calculated the Euclidean distance from the centroid of each ZCTA to the nearest centroid of a ZCTA within the same state that contained a provider location.
To assess whether an independent association exists between urban/rural status and EoE, after accounting for distance to provider, we used our previously published multivariable logistic regression model,(1) which adjusted for age, race, gender, ZCTA-level poverty, and county urban-rural code. We additionally adjusted for distance to provider, categorized into six levels. All analyses were performed in R version 4.0.2. Further details regarding the study methodology were previously reported(1) and can be found in the Online Repository Text.
A total of 18,452,886 children were included in these analyses, and the overall prevalence of EoE was 26.21/100,000, as was previously reported.(1) Distance to care provider was strongly associated with the NCHS classification of urban/rural status (Figure E1). In our previously published model, living in a rural area was associated with a lower risk of EoE diagnosis, when compared to living in a large-central metro area (Table 1). However, when adjusting for distance to provider, there was no longer an independent association between living in a rural area and EoE diagnosis (Table 1, Figure 1). In contrast, in this model, children living farther away from a pediatric gastroenterologist were less likely to be diagnosed with EoE, even after adjusting for urban/rural status and neighborhood-level poverty (26–50 km: aOR 0.84; 95%CI 0.75–0.93); 51–100 km: aOR 0.83; 95%CI 0.74–0.94; 101–200 km: aOR 0.68; 95%CI 0.59–0.79; >201 km: aOR 0.50; 95%CI 0.40–0.61; Table 1, Figure 1).
Table 1:
Association Between Poverty, Urban/Rural Status, Distance to Provider, and EoE
| Previous Model* | 95% CI | Updated Model† | 95% CI | |
|---|---|---|---|---|
| Sex | ||||
| Female | REF | REF | ||
| Male | 2.14 | 2.01 – 2.27 | 2.14 | 2.01 – 2.27 |
| Age Categories | ||||
| 0–2 y | REF | REF | ||
| 3–5 y | 0.99 | 0.90 – 1.09 | 0.99 | 0.90 – 1.09 |
| 6–8 y | 0.99 | 0.89 – 1.09 | 0.99 | 0.89 – 1.09 |
| 9–11 y | 1.03 | 0.94 – 1.14 | 1.04 | 0.94 – 1.14 |
| 12–14 y | 0.98 | 0.89 – 1.09 | 0.98 | 0.89 – 1.09 |
| 15–17 y | 0.87 | 0.77 – 0.98 | 0.87 | 0.77 – 0.98 |
| Race/Ethnicity | ||||
| White | REF | REF | ||
| Black | 0.41 | 0.38 – 0.45 | 0.40 | 0.37 – 0.44 |
| Asian | 0.37 | 0.28 – 0.49 | 0.36 | 0.27 – 0.47 |
| Hispanic | 0.31 | 0.27 – 0.35 | 0.30 | 0.27 – 0.34 |
| Grouped§ | 0.40 | 0.34 – 0.47 | 0.40 | 0.34 – 0.46 |
| Unknown | 1.64 | 1.48 – 1.80 | 1.61 | 1.46 – 1.77 |
| Urban/Rural Status | ||||
| Large Central Metro | REF | REF | ||
| Large Fringe Metro | 0.93 | 0.84 – 1.02 | 1.00 | 0.90 – 1.11 |
| Medium Metro | 0.95 | 0.87 – 1.05 | 1.04 | 0.94 – 1.15 |
| Small Metro | 0.78 | 0.69 – 0.88 | 0.95 | 0.82 – 1.09 |
| Micropolitan | 0.79 | 0.70 – 0.89 | 0.99 | 0.85 – 1.14 |
| Noncore (rural) | 0.68 | 0.59 – 0.78 | 0.88 | 0.74 – 1.03 |
| ZCTA-Level Poverty | ||||
| ≤ 5% | 2.00 | 1.75 – 2.28 | 1.99 | 1.74 – 2.28 |
| 6 – 10% | 1.78 | 1.57 – 2.00 | 1.79 | 1.58 – 2.02 |
| 11 – 15% | 1.50 | 1.33 – 1.69 | 1.53 | 1.36 – 1.73 |
| 16 – 20% | 1.30 | 1.14 – 1.49 | 1.31 | 1.15 – 1.50 |
| 21 – 25% | 0.99 | 0.84 – 1.16 | 0.99 | 0.85 – 1.16 |
| 26 – 100% | REF | REF | ||
| Distance to Provider | ||||
| ≤ 10 km | N/A | N/A | REF | |
| 11 – 25 km | N/A | N/A | 0.95 | 0.87 – 1.04 |
| 26 – 50 km | N/A | N/A | 0.84 | 0.75 – 0.93 |
| 51 – 100 km | N/A | N/A | 0.83 | 0.74 – 0.94 |
| 101 – 200 km | N/A | N/A | 0.68 | 0.59 – 0.79 |
| > 201 km | N/A | N/A | 0.50 | 0.40 – 0.61 |
Values expressed as odds ratios (OR) and 95% CIs
Previous models adjusted for sex, age, race/ethnicity, urban/rural status, state of residence, and ZCTA-level poverty
Updated model adjusted for the same variables as previous model, plus distance to provider
Defined as the combination of “Native American/Alaskan,” “>1 race (Hispanic), “>1 race (non-Hispanic),” and “Hawaiian”
ZCTA: Zip-code tabulation area
FIGURE 1.
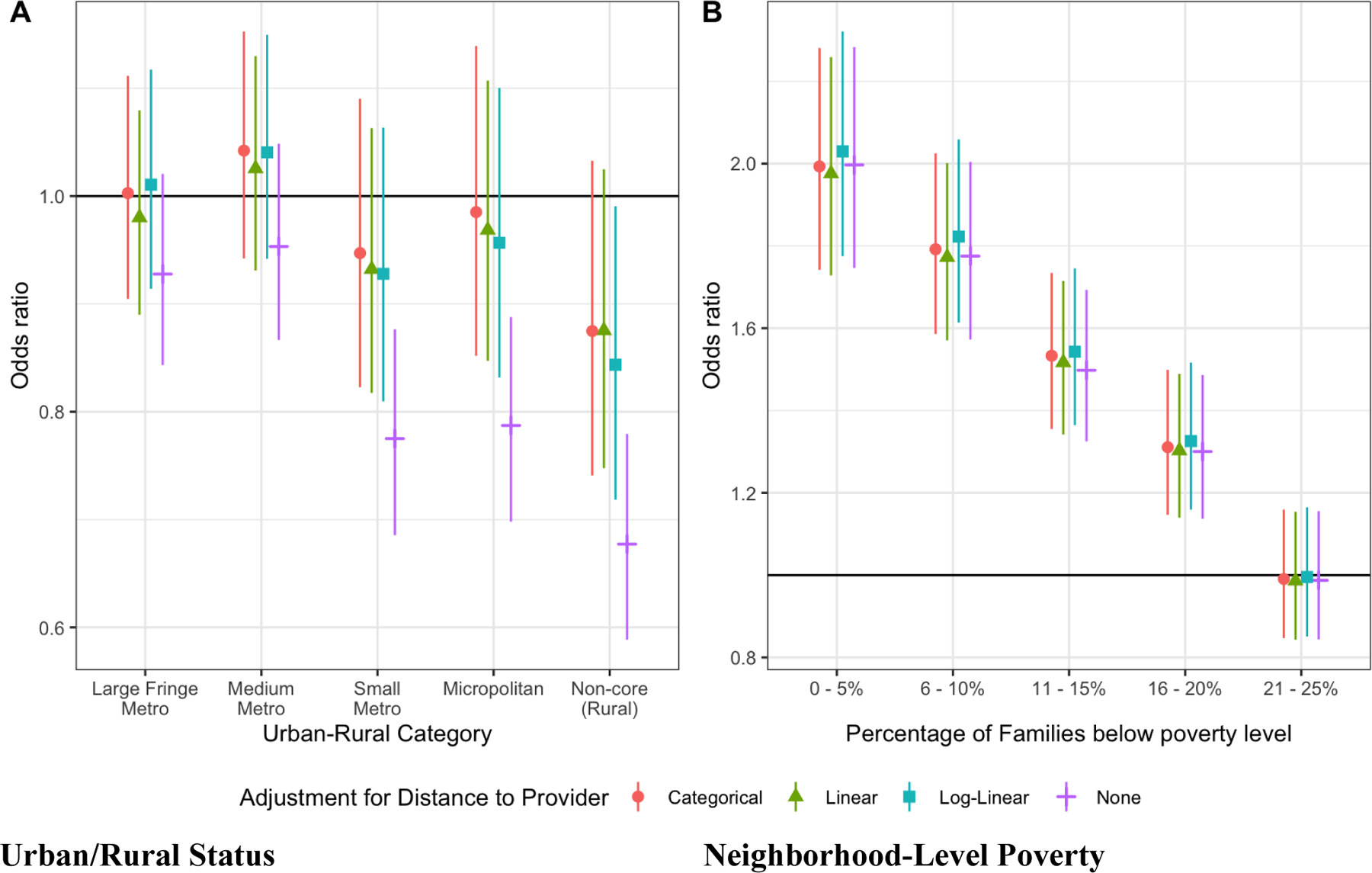
Odds ratios and 95% Cls for EoE diagnosis by (A) urban-rural county category and (B) neighborhood-level poverty. The reference group for the urban-rural analysis is Large central metro, and the reference group for neighborhood level poverty is less than 25% of families below the poverty line.
In our previous model, we had also observed an inverse relationship between poverty and EoE diagnosis, where children living in areas with higher neighborhood-level poverty had a lower odds of being diagnosed with EoE (Table 1). Distance to provider, however, was not strongly associated with neighborhood-level poverty in the cumulative distribution functions (Figure E1). Further, there was no attenuation of the strong inverse association observed between neighborhood-level poverty and odds of EoE diagnosis when distance to provider was included in the model (Table 1, Figure 1).
In this cross-sectional study of 18,452,886 children enrolled in Medicaid in 2012, we found that while living in a rural environment was associated with a decreased risk for EoE diagnosis, this protective effect was explained by distance to a pediatric gastroenterology provider. This strongly suggests that urban/rural disparities in EoE diagnosis are not primarily due to environmental factors, but are instead related to under-recognition that is associated with lack of access to subspecialty care. This study is the first to demonstrate that diagnostic disparities likely exist for the diagnosis of EoE among children living in rural areas. In order to diagnose EoE, a child must undergo an esophagogastroduodenoscopy (EGD) with esophageal biopsies,(3) which can only be performed by highly trained pediatric gastroenterologists. There are fewer pediatric gastroenterologists living in rural areas, and therefore these children do not have ready access to necessary providers. Unfortunately, previous studies have demonstrated that the delay in diagnosis of EoE is associated with an increased risk for fibrotic complications.(4) Increased access to pediatric gastroenterology providers or the development of non-invasive methodologies to diagnose EoE could minimize this health disparity and directly improve patient outcomes.
Interestingly, while distance to provider attenuated the association between urban/rural status and EoE diagnosis, this was not observed for neighborhood-level poverty. Children living in areas with higher neighborhood-level poverty continued to be less likely to be diagnosed with EoE. This findings suggests that either there are factors in areas with higher neighborhood-level poverty that decrease the risk for EoE, or there are other barriers to care that are not related to physical distance to a pediatric gastroenterology provider. Examples of such could include decreased access to specialty care among Medicaid participants,(5) the inability to take off work for appointments, and distrust in medical providers. Understanding these factors will improve our ability to identify these patients and provide care to those with untreated disease.
These findings further suggest that our current understanding of the epidemiology of EoE, which is based largely on administrative coding,(6) pathology databases,(7) and cohort studies(8) may not fully capture the predictors and phenotypic variability of this disease. While administrative coding has a very high specificity (99%), it has poor sensitivity (37–61%) even for those with diagnosed disease and likely does not capture all individuals with this condition.(9) Pathology database and cohort studies similarly only account for individuals who present for medical care and can be limited by selection bias. Further studies are needed to improve our understanding of the prevalence and predictors of EoE in high-risk and unselected populations.
This study is limited by the use of ICD-9 coding to define EoE, which, as above, has poor sensitivity for identifying patients with this condition.(9) Furthermore, these data were collected prior to the revised EoE diagnosis guidelines,(3) and thus the ICD-9 code may only have been used in patients who completed a PPI trial. This study is also limited by the lack of individual-level data on income, potentially leading to residual confounding. Finally, we were unable to assess whether patients in rural areas had fewer pediatric gastroenterology visits or EGDs, which would have corroborated our findings. These limitations are balanced by the fact that this study included over 18 million children enrolled in a nationwide database and provides novel data on likely diagnostic disparities in children with EoE.
Supplementary Material
FIGURE El. Empirical cumulative distribution function of distribution to provider by (A) urban-rural continuum code and (B) ZCTA-level poverty.
Clinical Implications:
In this cross-sectional study of children enrolled in Medicaid 2012, the apparent lower prevalence of EoE in rural communities was attenuated when adjusting for distance to provider. Diagnostic disparities likely exist for EoE among children residing in rural areas.
Acknowledgements:
CEGIR (U54 AI117804) is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including American Partnership for Eosinophilic Disorders (APFED), Campaign Urging Research for Eosinophilic Diseases (CURED), and Eosinophilic Family Coalition (EFC).
Funding:
This work was funded by the NIH through the following grants: (NIAID) 1K23AI103187, (NIAID) 1K23AI123596, Johns Hopkins Pediatrics Innovation Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: E. McGowan receives grant support from the National Institutes of Health (NIH). J. Keller and E.S. Dellon do not report any conflicts that are relevant to this paper. R. Peng receives grant support from the NIH and serves as a consultant for Health Effect Institute. C.A. Keet receives grant support from the NIH. E.T. Jensen receives grant support from NIH, the Kate B. Reynolds Foundation, and Optum Foundation.
Contributor Information
Emily C. McGowan, University of Virginia School of Medicine, Division of Allergy and Immunology, Charlottesville, VA; Johns Hopkins University School of Medicine, Division of Allergy and Clinical Immunology, Baltimore, MD.
Joshua P. Keller, Colorado State University, Department of Statistics, Fort Collins, CO.
Amanda B. Muir, Children’s Hospital of Philadelphia, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Philadelphia, PA.
Evan S. Dellon, University of North Carolina School of Medicine, Division of Gastroenterology, Chapel Hill, NC.
Roger Peng, Johns Hopkins Bloomberg School of Public Health, Department of Biostatistics, Baltimore, MD.
Corinne A. Keet, Johns Hopkins University School of Medicine, Division of Pediatric Allergy and Immunology, Baltimore, MD.
Elizabeth T. Jensen, Wake Forest School of Medicine, Department of Epidemiology and Prevention, Winston-Salem, NC.
References
- 1.McGowan EC, Keller JP, Dellon ES, Peng R, Keet CA. Prevalence and geographic distribution of pediatric eosinophilic esophagitis in the 2012 US Medicaid population. J Allergy Clin Immunol Pract. 2020;8(8):2796–8.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabet C, Klion AD, Bailey D, Jensen E, Chehade M, Abonia JP, et al. Do rural health disparities affect prevalence data in pediatric eosinophilic esophagitis? J Allergy Clin Immunol Pract. 2021;9(6):2549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated International Consensus Diagnostic Criteria for Eosinophilic Esophagitis: Proceedings of the AGREE Conference. Gastroenterology. 2018;155(4):1022–33.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–6.e1–2. [DOI] [PubMed] [Google Scholar]
- 5.Bisgaier J, Rhodes KV. Auditing access to specialty care for children with public insurance. N Engl J Med. 2011;364(24):2324–33. [DOI] [PubMed] [Google Scholar]
- 6.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12(4):589–96.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson J, O’Gorman M, McClain A, Mutyala K, Davis C, Barbagelata C, et al. Incidence and Prevalence of Pediatric Eosinophilic Esophagitis in Utah Based on a 5-Year Population-Based Study. Clin Gastroenterol Hepatol. 2019;17(1):107–14.e1. [DOI] [PubMed] [Google Scholar]
- 8.Franciosi JP, Tam V, Liacouras CA, Spergel JM. A case-control study of sociodemographic and geographic characteristics of 335 children with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7(4):415–9. [DOI] [PubMed] [Google Scholar]
- 9.Robson J, Korgenski K, Parsons K, McClain A, Barbagelata C, Allen-Brady K, et al. Sensitivity and Specificity of Administrative Medical Coding for Pediatric Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2019;69(2):e49–e53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE El. Empirical cumulative distribution function of distribution to provider by (A) urban-rural continuum code and (B) ZCTA-level poverty.


