Figure 6.
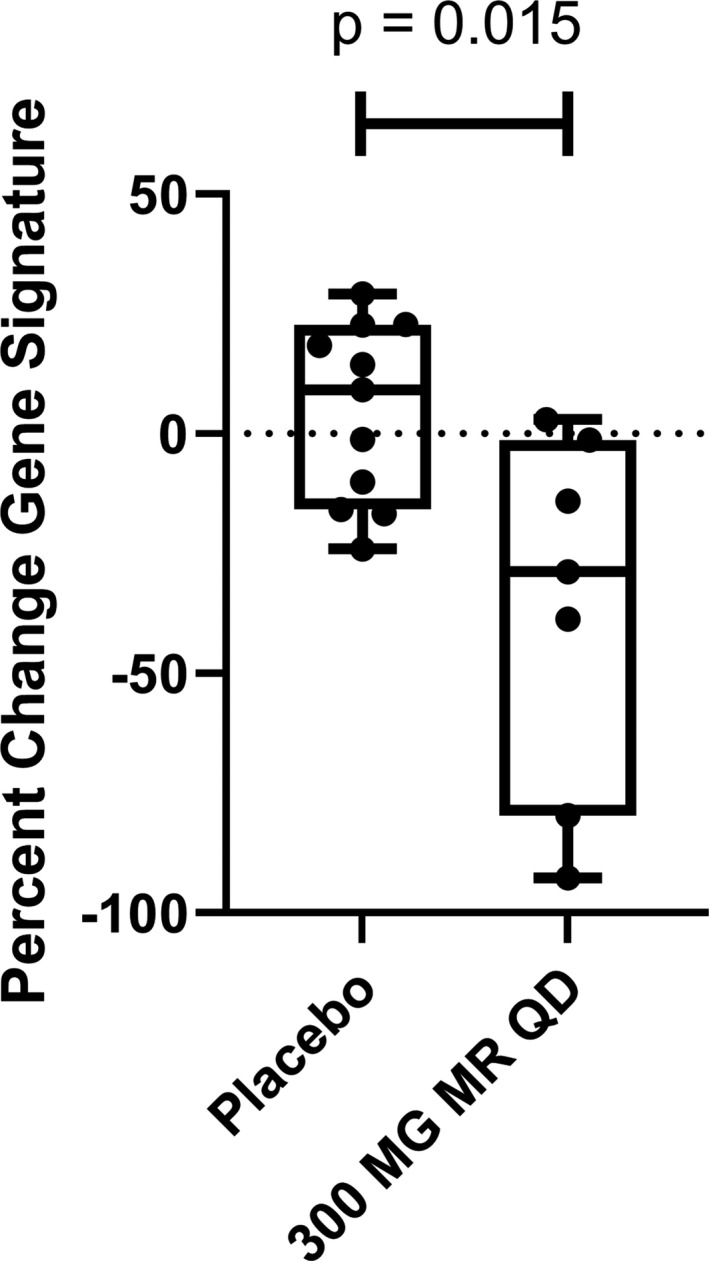
PF‐06650833 inhibits type I interferon signature in vivo in humans. Moderate‐release (MR) PF‐06650833 (300 mg/day [qd]) (n = 7) or placebo (n = 11) was administered for 14 days in a phase I multiple‐ascending‐dose trial in healthy human volunteer subjects. Whole blood was collected in a PAXgene tube on day 0 prior to administration of the first dose and on day 14 prior to administration of the last dose, RNA extracted, and a composite gene signature calculated. The percent change in the composite gene signature for each individual participant between the 2 time points is shown. Each box represents the 25th to 75th percentiles. Lines inside the boxes represent the median. Lines outside the boxes represent the full range of values. Symbols represent individual patients.
