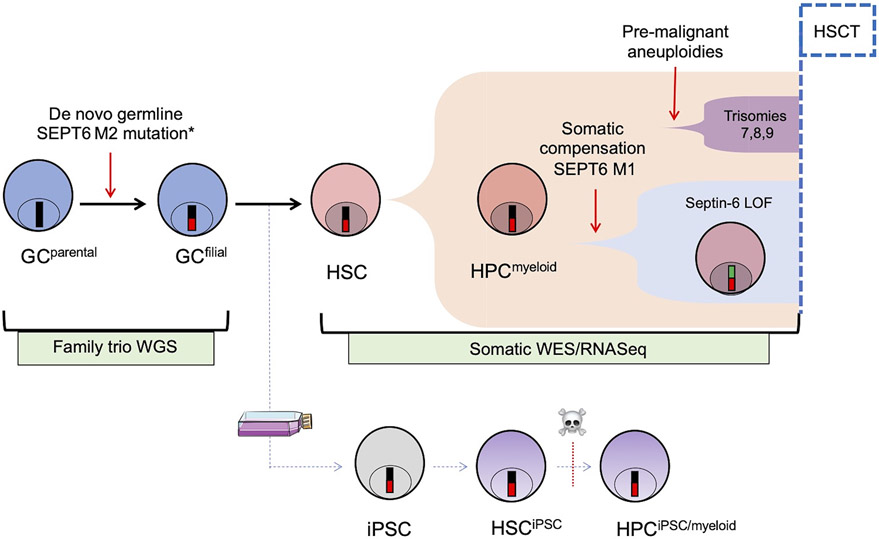
Figure 1: Schematic representation of the study.
A newborn with severe congenital neutropenia developed progressive MDS with tetraploid myeloid progenitors, and had to undergo an allogeneic HSCT before age 1 y.o. He was found to carry a novel de novo germline mutation in the C-terminus of SEPT6 (mutation M2), not identified in the trio whole-genome/whole-exome NGS (WGS/WES) analysis of his biological parents. SEPT6 is located on the X chromosome and thus the mutation hemizygous in this boy (*). This mutation was associated with the accumulation of additional pre-malignant clonal aberrations (trisomy 7,8,9) and progressive tetraploidy in the patient’s BM. By WES of the patient’s pre-HSCT BM, we also we also identified a somatically acquired, low-frequency compensatory frameshift SEPT6 mutation (M1). Early HSCT was performed to treat the progressive cytopenias and to prevent transformation from MDS to AML. The patient is alive and well without any other hematological or other phenotype 10 years after HSCT. To dissect the putative role of the SEPT6-M2 mutation, we generated fibroblast-derived iPSCs from the patient and controls and studied them and their hematopoietic progeny. LOF = loss of function.