Figure 1. CSF-1, GM-CSF and G-CSF receptors and their main downstream signaling pathways.
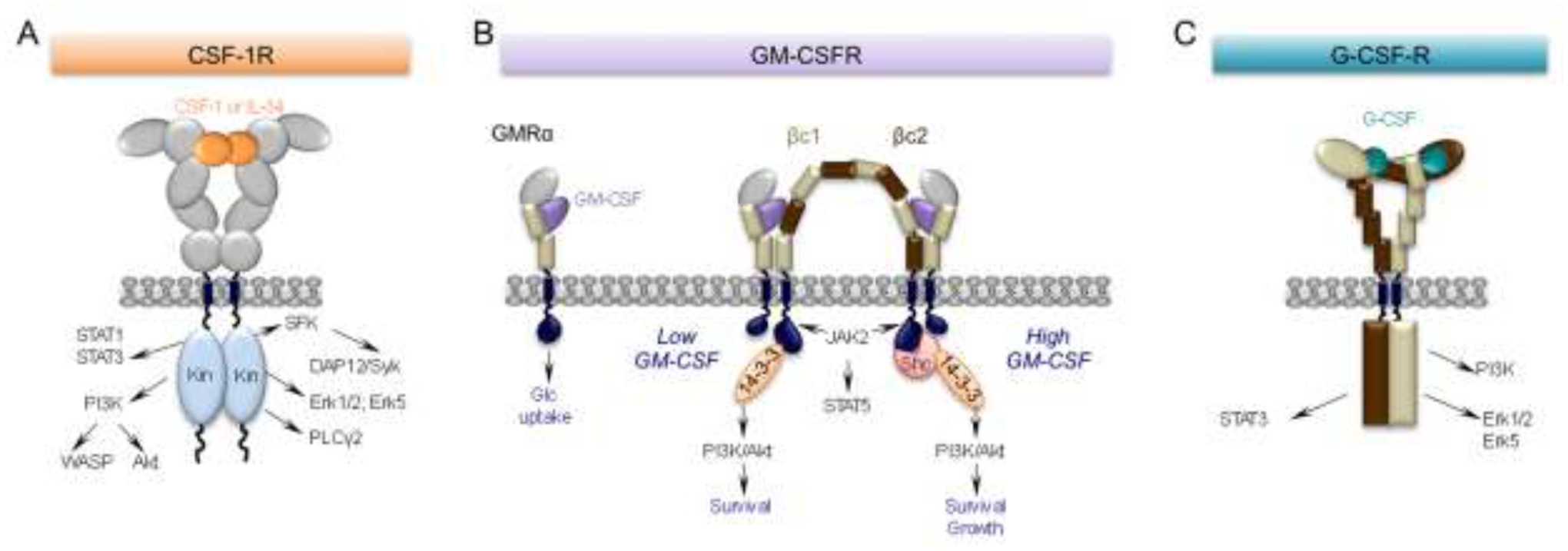
(A) The CSF-1R is a homodimeric tyrosine kinase that binds homodimers of CSF-1 or IL-34. The extracellular domain consists of five immunoglobulin (Ig)-like domains. Ligand binding triggers downstream tyrosine phosphorylation, initiating a cascade of signaling events resulting in cell differentiation, survival, proliferation, migration and suppression of inflammation (reviewed in [2]). (B) Monomeric GM-CSF binds with relatively low affinity (nM range) to a “wrench-like” structure formed by the N-terminal Ig domain and the 2 fibronectin type III domains of the GMRα chain (GMRα). Even in the absence of the common β subunit (βc), this interaction is sufficient to elicit a biological response, i.e. glucose uptake [37]. In cells also expressing βc, the GM-CSF/GMRα complexes further associate with the β chains, creating a hexameric high-affinity receptor (100pM range) with the depicted structure. These complexes associate laterally through the GMRα subunit to form a dodecameric complex that is responsible for signaling (reviewed in [16]). Mutually exclusive phosphorylation events at βc residues, S585 or Y577, recruit either the 14–3-3 adaptor protein at low concentrations of GM-CSF (S585) or, in the setting of high GM-CSF concentrations, Shc (Y577), mediating a molecular switch between survival and survival and growth, respectively. (C) G-CSF is monomeric. The G-CSF receptor has six extracellular domains (D1-D6). D1 is an N-terminal Ig-like domain and D2-D6 are fibronectin type III domains. These are followed by a transmembrane domain and an intracellular domain without intrinsic kinase activity. D2 and D3 form the cytokine receptor homologous (CRH) module involved in ligand binding, while D4-D6 facilitate dimerization of the cytoplasmic regions. The signaling unit is a 2:2 receptor:ligand complex, in which each G-CSF monomer binds one receptor through the CRH module and the second receptor through the Ig domain (reviewed in [40]). In neurons, ligand binding activates the Erk family and enhances neuronal survival while activation of the PI3-K/Akt and STAT3 signaling pathways and prevents apoptotic cell death, by inhibiting activation of caspases and by increasing anti-apoptotic protein members such as Bcl-xL (reviewed in [42]).
