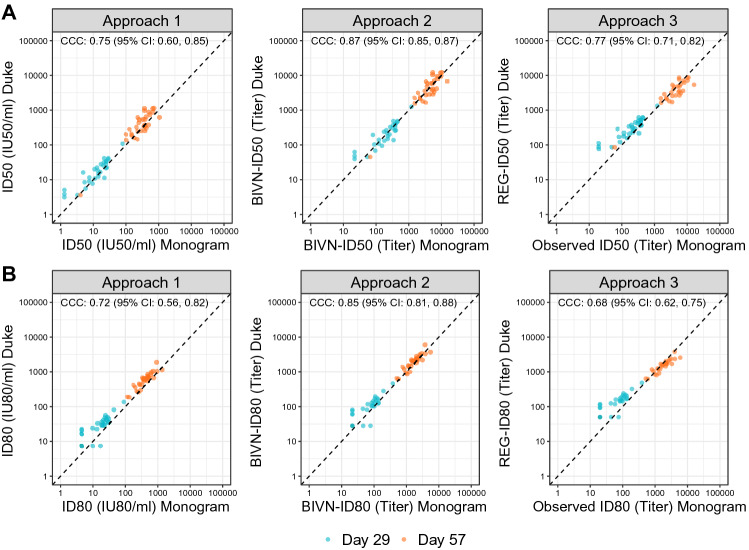
Figure 3.
Scatterplots of calibrated ID50 (Panel A) and ID80 (Panel B) titers from the Duke and Monogram labs demonstrating performance of the three calibration approaches using vaccine recipient samples collected at Day 29 (turquoise) and Day 57 (orange), 4 weeks post the first and the second mRNA-1273 vaccine doses, respectively. The arithmetic mean-based calibration factor was used in Approach 1. The concordance correlation coefficient (CCC) and 95% confidence intervals indicate the level of agreement between the x- and y-axis values. ID50, ID80: ID50, ID80 titers calibrated to the WHO International Standard, expressed in International Units per ml (IU50/ml for ID50; IU80/ml for ID80) (Approach 1). BIVN-ID50, BIVN-ID80: ID50, ID80 titers from each lab calibrated to a common scale using an independent pool of clinical samples, assuming a bivariate normal distribution for the readouts from the two labs (Approach 2). REG-ID50, REG-ID80: ID50, ID80 titers from the non-reference lab (Duke) calibrated to the reference lab (Monogram) using an independent pool of clinical samples, based on regressing titers from the reference lab on the non-reference lab (Approach 3).