Abstract
Tuberculosis is ancient disease known to mankind. Diagnosis and management of spinal tuberculosis has immensely improved in last few decades. Imaging, particularly MRI, plays important role in diagnosis of spinal tuberculosis and its complications. Four common imaging patterns of spinal tuberculosis include paradiscal type, central type, Anterior subligamentous type, and posterior type. Imaging also plays important role in differentiation of spinal tuberculosis from its mimics, particularly pyogenic spondylitis, and metastasis. Radiological interventions, such as CT guided vertebral biopsy, and percutaneous drainage of cold abscess, are commonly used in management of spinal tuberculosis. Monitoring of therapeutic response is often based on clinical evaluation and imaging. MRI is most common imaging modality used. Signs of healing include bony ankylosis, resolution of marrow edema, decrease in contrast enhancement, and fatty change with in bone marrow. PET CT is recently evaluated for response assessment with promising results. This review summarizes pathophysiology, clinical presentation, imaging features, radiological interventions, and response assessment in spinal tuberculosis.
Keywords: Spinal tuberculosis, Spinal infection imaging, Imaging intervention, Response evaluation
1. Introduction
Tuberculosis is one of the ancient diseases known to mankind.1 It is observed in mummies of Egypt and Peru, as old as 9000BCE. In India, it has been mentioned in Rig Veda, and Atharva Veda, by the name ‘Yakshama’ as early as in 3500BCE–1800BCE. In 1779, Sir Percival Pott observed spinal tuberculosis as a disease causing spinal deformity and paraplegia.1,2 It was in 1870, that the mycobacterium was identified as the causative agent.3 Since then, diagnosis and management of tuberculosis has improved immensely. More recently, since 1987, availability of computed tomography (CT) and magnetic resonance imaging (MRI) has made it possible to diagnose spinal tuberculosis in a pre-destructive phase, and at rare and difficult sites such as craniovertebral junction.3 Thus, presently, with availability of advanced imaging techniques, and effective antitubercular drugs, the objective of the treatment of spinal tuberculosis is ‘to eradicate the infection with near normal spine’.4 Another concern in the treatment of spinal tuberculosis is the decision on the optimum duration of antitubercular therapy. Although, world health organization (WHO) and American Thoracic Society recommend category I antitubercular therapy for 6–9months in newly diagnosed spinal tuberculosis, stopping antitubercular therapy at fixed 6–9 months duration may not be ideal, considering potentially disastrous complications of spinal tuberculosis. It is recommended that clinical response along with imaging be used to decide the end point of antitubercular therapy.5 MRI and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET), are particularly useful in evaluating therapeutic response.6,7 This review summarizes pathophysiology, clinical presentation, imaging features, radiological interventions, and response assessment in spinal tuberculosis.
2. Pathophysiology of spinal tuberculosis
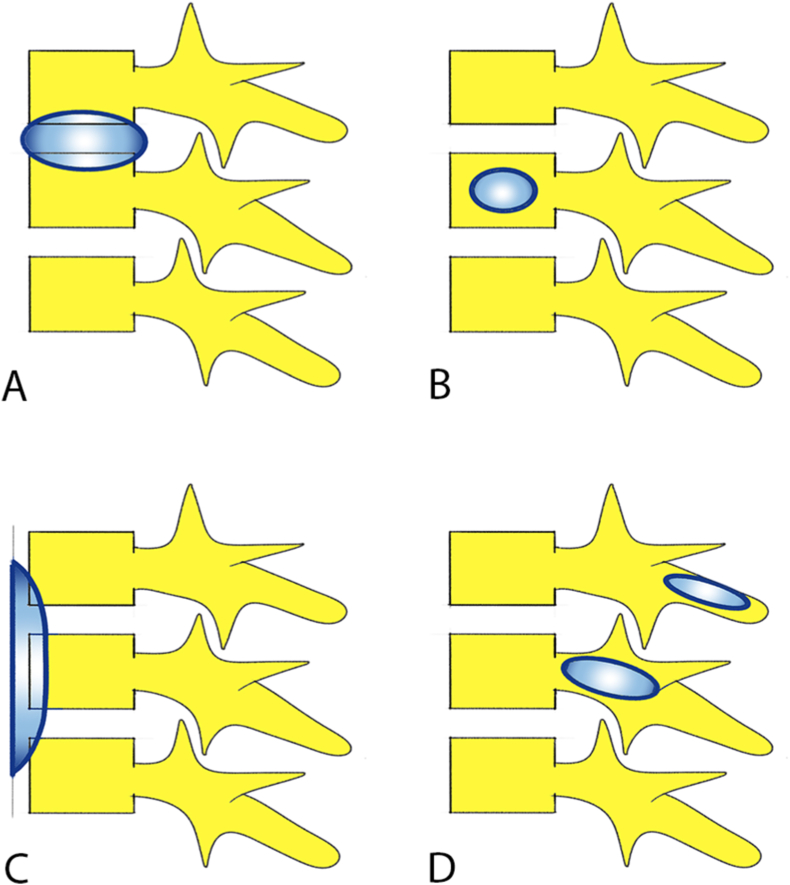
Tuberculosis is caused by slow growing aerobic bacilli, mycobacterium tuberculosis complex.2 Lung is the most common site of primary infection. Spinal involvement is always secondary to hematogenous dissemination of bacilli from the primary site.2 Thoracolumbar junction is the most common site involved.8 Four patterns of vertebral involvement in spinal tuberculosis8,9 include (Fig. 1).
-
i)
Paradiscal type where vertebral bodies, adjacent to the intervertebral disc, are involved secondary to arterial spread of infection. It is the most common type.
-
ii)
Central type where central vertebral body is involved from spread of infection through valveless Batson's paravertebral venous plexus.
-
iii)
Anterior type results from subligamentous spread of infection beneath the anterior longitudinal ligament.
-
iv)
Posterior element involvement in spinal tuberculosis is rare, and is caused by spread of infection through posterior external vertebral venous plexus or by direct spread.8,9
Fig. 1.
Patterns of vertebral involvement in spinal tuberculosis. (A: Paradiscal; B: Central; C: Anterior subligamentous; D: Posterior (neural arch) involvement).
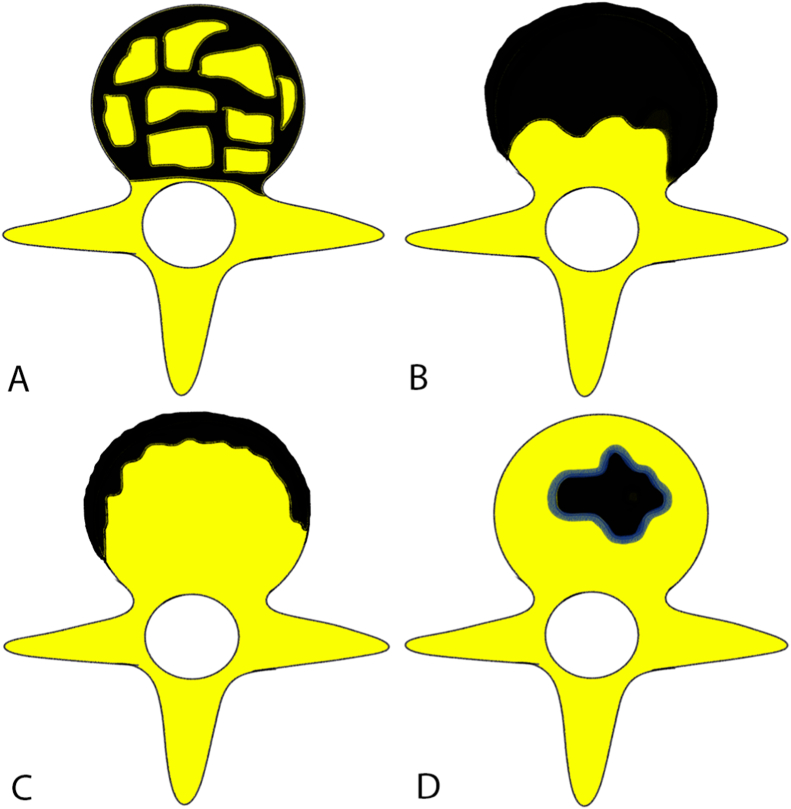
Intervertebral disc is a relatively avascular structure, and is spared until late stage of the disease, as tuberculous bacilli do not produce proteolytic enzymes, in contrast to pyogenic infection where it is involved early in disease.2 Bone destruction in spinal tuberculosis can be fragmentary (47%), osteolytic (34%), subperiosteal (30%), or localized destruction with sclerosed margins (10%)10 (Fig. 2). Bone destruction will eventually lead to anterior wedging and kyphosis or concertina collapse of single vertebral body. Disc osseous debris with in the spinal canal and epidural pus can compress on the spinal cord or the nerve roots and cause neurological sequelae.10 Cold abscesses are pus collections that lack surrounding inflammatory response, and are seen in nearly 70% of patients with spinal tuberculosis. It is often seen in paravertebral region, and can take the path of least resistance along the fascial planes and neurovascular bundle.2,9
Fig. 2.
Bone destruction types in spinal tuberculosis. (A: Fragmentary; B: Osteolytic; C: Subperiosteal; D: Localized destruction with sclerosed margins).
Late onset paraplegia can occur even after resolution of active infection in the presence of persistent severe kyphosis. This can lead to chronic compression over spinal cord and eventually myelomalacia which is irreversible and results in permanent neurological deficits. Thus, early control or surgical correction of kyphosis is necessary to prevent permanent functional disability.10
3. Clinical features
Spinal tuberculosis shows increasing trending in recent years, and largest number of patients belong to the age group of 16–30 years.11 Spinal tuberculosis can have varied presentations. Often, it is insidious in onset with long duration of history. Uncomplicated spinal tuberculosis is where the diagnosis is made before development of complications. Back pain is the most common presentation, seen in 90%–100% of spinal tuberculosis, and is the only complaint in 61% of patients.9,11 Constitutional symptoms such as fever, malaise, loss of appetite, and loss of weight which are common in pulmonary tuberculosis, are infrequent in spinal tuberculosis (20%–30%). Complicated spinal tuberculosis can present with deformity, instability, or neurological deficits.2,9
Patients without constitutional symptoms, back pain, kyphosis, or typical imaging features are said to have atypical spinal tuberculosis.2 Common examples for atypical presentation on imaging include skip vertebral lesions from spread of infection through Batson's vertebral venous plexus, concertina collapse, multifocal involvement, isolated neural arch involvement, isolated cold abscess, and prolapsed intervertebral disc.2
4. Diagnosis of spinal tuberculosis
Diagnosis of spinal tuberculosis is often suggested by characteristic clinical features, and imaging findings. WHO recommends use of Tuberculin skin test in low income countries, for its high negative predictive value. However, its use is limited, particularly in endemic countries, as it cannot differentiate between latent from active infection, and also, the test can be falsely negative in immunocompromised individual.2,12 Tissue diagnosis is the gold standard for diagnosis of spinal tuberculosis, and bacteriological confirmation can be obtained by culture, histopathology, or polymerase chain reaction (PCR).9
5. Imaging in spinal tuberculosis
-
1)
Plain radiograph:
Anteroposterior and lateral spine radiographs are often the initial screening investigations done in evaluation of back pain or suspected spinal infection.5 Though the spine radiograph is rudimentary in the diagnosis of spinal tuberculosis, it forms the cornerstone in diagnosis of spinal disease in resource poor endemic countries. The findings become apparent on radiograph when at least one-third of calcium is lost from bones. Often, by the time the spinal abnormalities are seen on radiograph, the disease is in advanced stage with deformity, or neurological deficits. Also, it is difficult to see involvement of certain sites such as craniovertebral junction, and cervicodorsal junction, on radiograph.9,12,13
Paradiscal type is most common type.9,12 Earliest finding that is seen on radiograph is decreased bone density and loss of definition of vertebral endplates. Other common findings that are seen on radiograph are end plate erosions, vertebral geode, disc space loss, bone destruction, bone sequestration, vertebral height reduction, and paravertebral soft tissue (Fig. 3). Sclerosis is unusual prior to institution of therapy. Central type is characterized involvement of single vertebral body and concertina collapse (Fig. 4).9,12 Subligamentous spread of infection causes scalloping and erosion of anterior vertebral margin (giving appearance of aneurysmal syndrome).13 On radiograph, cold abscesses are seen as peri-vertebral soft tissue densities. In cervical spine, it can cause widening of retropharyngeal space on lateral cervical radiograph. In upper dorsal spine, it can appear as superior mediastinal widening on anteroposterior radiograph, and prevertebral soft tissue indenting on trachea on lateral radiograph. In lower dorsal and lumbar spine, cold abscesses are seen as paravertebral soft tissue. Calcification in paravertebral soft tissue is characteristic for spinal tuberculosis.12
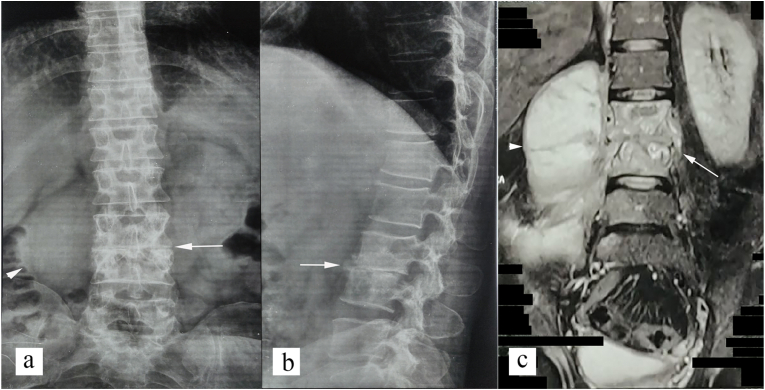
Fig. 3.
Radiographic features of spinal tuberculosis (paradiscal type). (a) anteroposterior and (b) lateral radiograph of lumbar spine shows loss of L3-L4 disc space, endplate erosions, lytic destruction of L3 and L4 vertebral bodies (white arrow), and right paravertebral soft tissue (white arrowhead). (c) coronal STIR image shows loss of L3-L4 disc space, marrow edema in L3, L4 (white arrow), and also L5 vertebral bodies, and right psoas abscess consistent with spinal tuberculosis (white arrowhead).
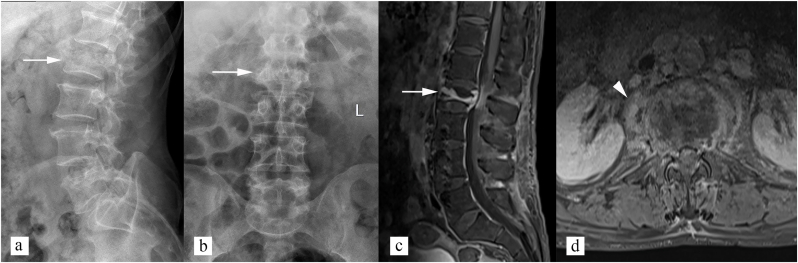
Fig. 4.
Radiographic features of spinal tuberculosis (central type). (a) Lateral and (b) Anteroposterior radiograph of lumbar spine shows isolated L2 vertebral involvement with concertina collapse of vertebral body (white arrow). No paravertebral soft tissue appreciated on radiographs. (c) sagittal post contrast T1 weighted image shows abnormal enhancement of L2 vertebral body (white arrow). (d) axial post contrast T1 weighted image shows enhancing paravertebral soft tissue (white arrowhead).
Chest radiograph is recommended in patients with spinal tuberculosis. Nearly 67% of patients with spinal tuberculosis either have active pulmonary tuberculosis or have had pulmonary tuberculosis in the past.12
-
2)
Computed tomography (CT):
CT shows changes of spinal tuberculosis earlier than radiographs. Bone destruction is better appreciated on CT, and can be fragmentary (47% of cases), osteolytic (34%), subperiosteal (30%), or localized destructive with sclerotic margins (10%).12 CT can also demonstrate paravertebral abscess, enhancing granulation tissue, and spinal canal encroachment by bone fragments, disc debris, or pus. Over 60% of patents with spinal tuberculosis have spinal canal encroachment.9,13 CT is most sensitive in detecting calcification in paravertebral soft tissue which is pathognomonic of tuberculosis (Fig. 5). Multidetector CT technology, and multiplanar reconstruction allow detailed evaluation of spinal infection and deformity, which is essential for surgical planning. New techniques like virtual non-calcium CT based on Dual Energy CT, can detect bone marrow edema, and thus, have potential to detect disease early in its pre-destructive stage, and also, collagen mapping in dual energy CT have potential to detect intervertebral disc involvement in spinal infection.14 Dual energy CT can also be useful in differentiation of spinal osteolytic metastasis from spinal infection.15 CT is also the preferred imaging modality for percutaneous vertebral biopsy for tissue sampling and confirmation of the disease.9,12,13 Helical CT can also be used to create 3D printed models of tuberculous spine, and personalized pedicle guide plates, useful in surgical planning. This is shown to reduce the amount of blood loos, operation and fluoroscopy time, and also reduce the complications associated with nail placement.16
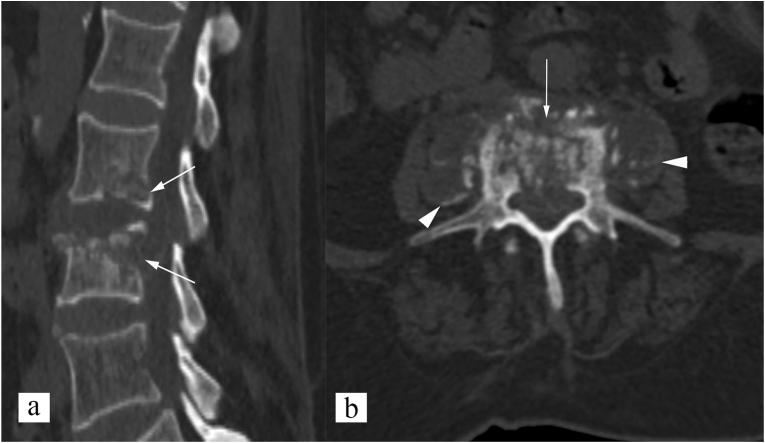
Fig. 5.
CT features of spinal tuberculosis (paradiscal type). (a) sagittal non-contrast CT image shows paradiscal end plate erosions, and vertebral body involvement of L2 and L3 vertebrae (white arrows). (b) axial non-contrast CT image shows fragmentary type bone destruction (white arrow), and paravertebral soft tissue (white arrowhead). Calcification is noted in paravertebral soft tissue, which is pathognomonic for spinal tuberculosis.
-
3)
Magnetic resonance imaging (MRI):
Magnetic resonance imaging is the best imaging modality for evaluation of spinal tuberculosis, with reported sensitivity of 96%, and specificity of 93%.17,18 Infectious disease society of America (IDSA) strongly recommends MRI in evaluation of suspected spinal infection.17 MRI is indispensable for evaluation of spinal canal compromise and cord compression in patients with neurological deficits.9 Some centers recommend whole spine MRI at the time of initial diagnosis or suspicion of spinal tuberculosis.9 It has two major advantages. First, it allows early identification of disease process even before bone destruction, deformity or disability from deformity has occurred. Second, it is useful to identify skip lesions, particularly those which are not readily apparent on radiograph. It is particularly important, as the involved vertebrae can collapse as a natural course of disease, even when on chemotherapy. This can be wrongly interpreted as new abnormality when patient is just followed on radiograph, and can lead to false diagnosis of multidrug resistance tuberculosis.9
MRI signal changes can be observed as early as 3–5 days after spinal infection, when no other imaging modality shows any abnormality.13,17 All the four patterns of spinal tuberculosis, namely, paradiscal type (Fig. 6), central type (Fig. 7), anterior subligamentous type (Fig. 8), and posterior type (Fig. 9), can easily be identified on MRI. In spinal tuberculosis, MRI often shows inflammatory marrow edema as hypo-intensity on T1 weighted images and hyper-intensity on short tau inversion recovery (STIR) and T2 weighted images.6,13 Contrast enhancement improves the diagnostic accuracy of detection of spinal tuberculosis, and is useful in evaluating extent of disease, and disease activity. Well defined and heterogeneous enhancement is seen in the region of inflammatory marrow edema, on post gadolinium images.9,19 Disc involvement is seen later in the course of disease and is seen as reduced disc space, high signal intensity on T2 weighted image with loss of intranuclear T2 hypointense cleft, and contrast enhancement.13 Both phlegmon/granulation tissue and cold abscess appear as high signal intensity on T2 weighted images, and low signal intensity on T1 weighted images. However, on contrast enhanced MRI, phlegmon shows uniform enhancement, and abscess shows smooth, thin peripheral wall enhancement.13,19 Thoracolumbar spine is the most common site affected and paraspinal abscess can track along iliopsoas muscles to reach into the retroperitoneum and pelvis. MRI can also demonstrate intramedullary or extramedullary tuberculomas, spinal cord edema, and myelomalacia.13 Atypical presentation on imaging include multifocal involvement (Fig. 10), skip vertebral lesions, isolated cold abscess (Fig. 11), and prolapsed intervertebral disc2
Fig. 6.
MRI findings in spinal tuberculosis, paradiscal type. (a) Sagittal STIR image, and (b) sagittal post contrast T1 weighted image shows reduced disc space, with inflammatory marrow edema of D10 and D11 vertebral bodies seen (white arrow). (c) Coronal post contrast T1 weighted image shows bilateral paravertebral peripherally enhancing cold abscesses (white arrowhead).
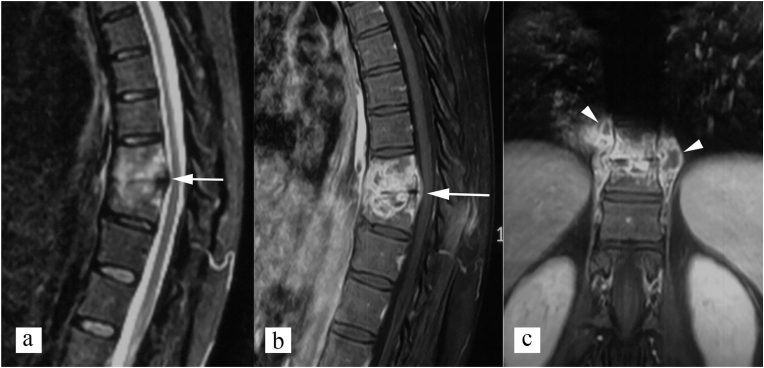
Fig. 7.
Central type of spinal tuberculosis. (a) STIR sagittal, and (b) post contrast T1 weighted sagittal MR images show inflammatory bone marrow edema involving D2 vertebral body (white arrow), epidural abscess compromising the spinal canal (black arrowhead), and prevertebral abscess (white arrowhead).
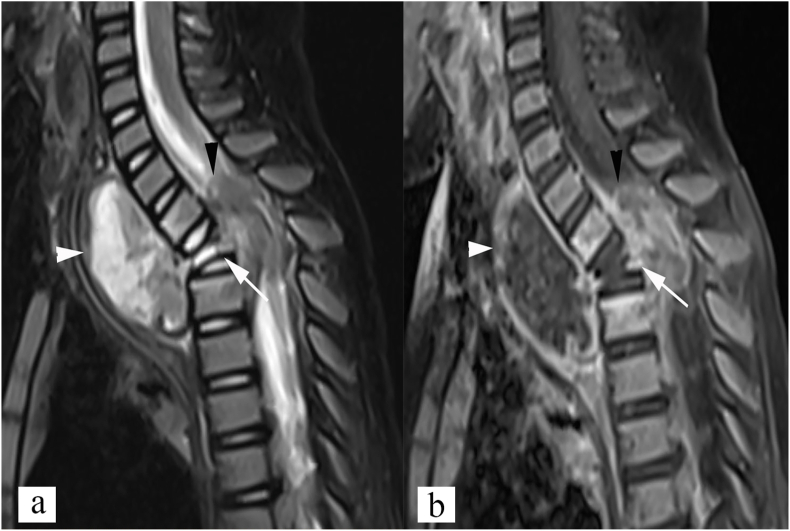
Fig. 8.
Anterior subligamentous type of spinal tuberculosis. Sagittal STIR (a), and post contrast T1 weighted (b) images show subligamentous abscess (white arrow) beneath the anterior longitudinal ligament along L5, and sacral vertebrae, causing scalloping of anterior vertebral margins (white arrow). In addition, inflammatory marrow edema also noted in L5, and sacral vertebral bodies, adjacent to the abscess (white arrowhead).
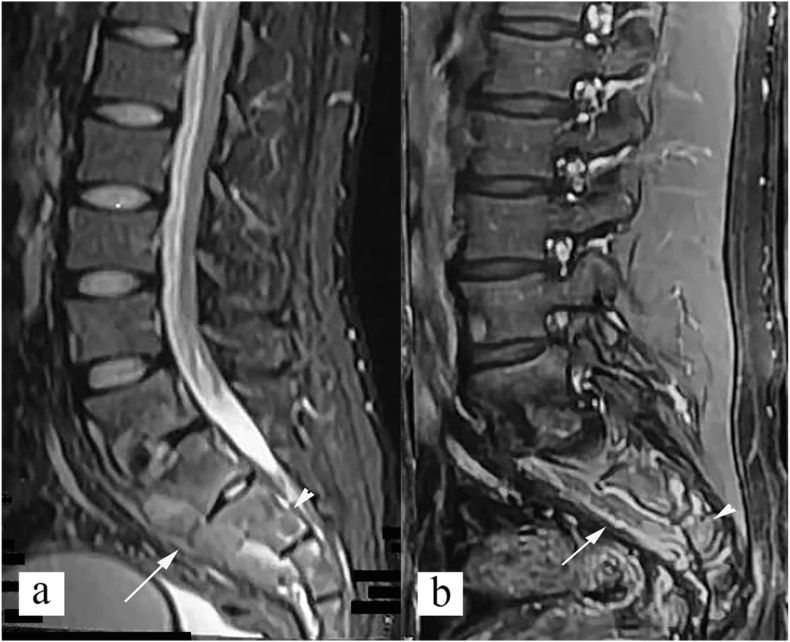
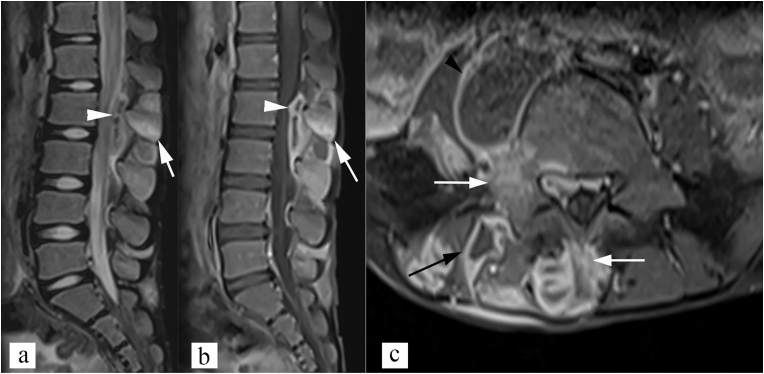
Fig. 9.
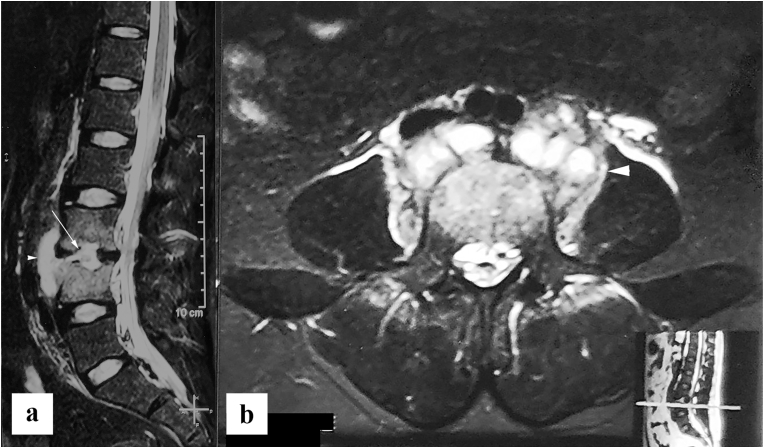
Posterior type of spinal tuberculosis. (a) Sagittal STIR image, and (b) sagittal post contrast T1 weighted image show inflammatory marrow edema in L2 and L3 spinous process (white arrow), with epidural abscess (white arrowhead). Note that vertebral bodies shows normal marrow signal. (c) axial post contrast T1 weighted image shows involvement of spinous process, and right lamina, and pedicle (white arrow) with right posterior paravertebral abscess (black arrow) and right psoas abscess (black arrowhead).
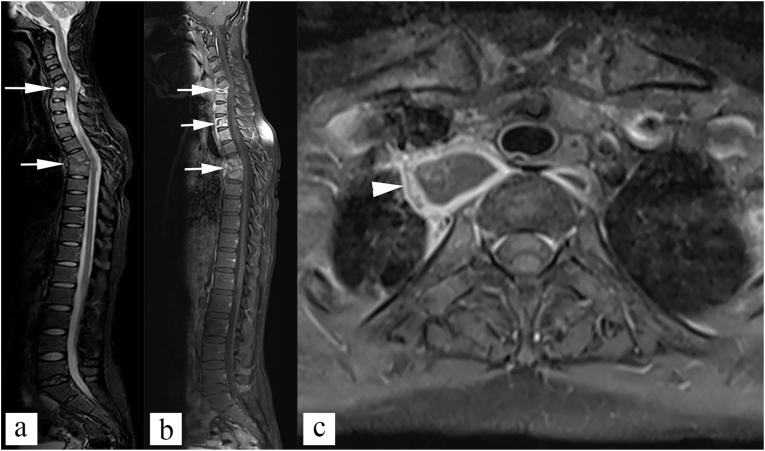
Fig. 10.
Multifocal disease. (a) Sagittal STIR image, and (b) sagittal post contrast T1 weighted image shows multiple cervical and upper dorsal vertebral body involvement (white arrows). Axial post contrast T1 weighted image shows paravertebral cold abscess (white arrowhead).
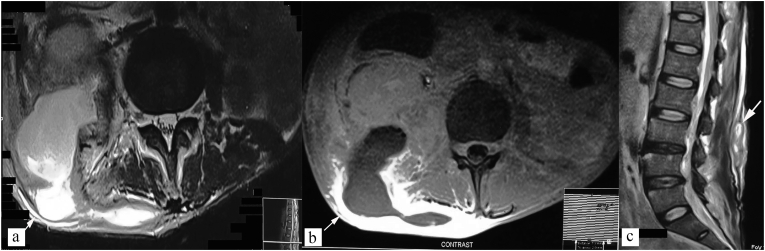
Fig. 11.
Atypical presentation - isolated cold abscess. Axial STIR (a), and T1 weighted post contrast (b) images show tubercular cold abscess in right posterior paraspinal location, posterior abdominal wall, and posterior pararenal space (white arrow). (c) Sagittal T2 weighted image shows normal vertebra marrow signal intensity.
MRI findings that show high sensitivity and specificity in differentiation of spinal tuberculosis from non-tubercular lesions include subligamentous spread of infection to three or more contiguous vertebral levels, large abscess with smooth thin wall, and >50% vertebral destruction. Diagnostic accuracy further improves when combination of above findings are present.13,20
-
4)
Nuclear medicine studies:
Nuclear medicine studies can measure metabolic activity within the lesion, differentiate infectious from non-infectious pathology, identify multifocal disease, and assess response to treatment.13
Both 99mTc- Diphosphonate scan and 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) can identify abnormal metabolic activity at the site of active inflammation, and multifocal involvement (Fig. 12), however, they cannot very well differentiate between infectious and non-infectious pathology.21,22 Gallium67 is an inflammatory labelling radionuclide isotope that is particularly useful in identifying infection. Combined Gallium and 99mTc- Diphosphonates scan shows high sensitivity (90%) and specificity (78%) for identification of bone and soft tissue infection.13 PET CT may also be useful in selection of lesion or abscess with higher metabolic activity for tissue sampling that can improve the diagnostic yield.9,23
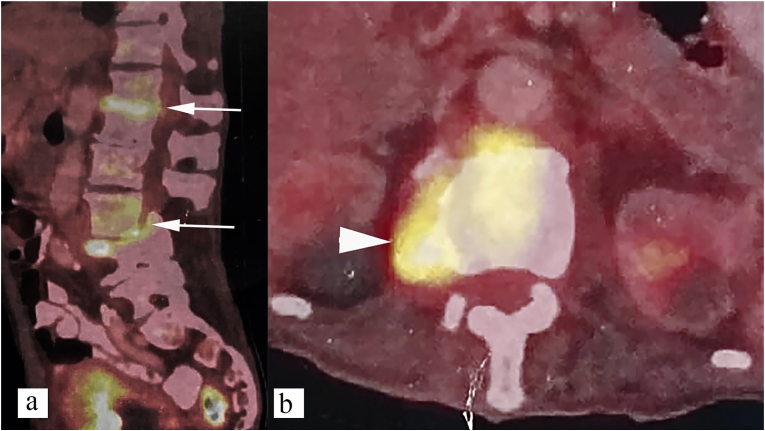
Fig. 12.
18F-FDG PET CT features of spinal tuberculosis (paradiscal type). Paradiscal increased metabolic activity seen at D12-L1 and L3-L4 vertebral levels (a) (White arrow), right paravertebral soft tissue (b) (white arrowhead).
6. Differential diagnosis
Few common conditions that mimic spinal tuberculosis include pyogenic spondylitis, brucellosis, Andersson's lesion in ankylosing spondylitis, SAPHO (Synovitis, Acne, Pustulosis, Hyperostosis, Osteitis) syndrome, rheumatoid arthritis, degenerative spine disease, baastrup's disease, Osteoporotic fracture, Neuropathic spine, and metastasis.24,25 Table 1 provides few salient features that can help differentiate spinal tuberculosis from above mentioned conditions. Often, diagnosis is based on combination of clinical features, imaging, and histopathology.
Fig. 13.
Pyogenic spondylitis (biopsy proven) in 30year old male patient with acute onset fever and back pain. (a) Sagittal, and (b) axial STIR images show reduced L3-L4 intervertebral disc space, with abnormally high T2 signal intensity with in the disc (white arrow), and thick irregular walled paraspinal abscess (white arrowhead). Bone marrow edema seen in L3 and L4 vertebral bodies with relatively preserved vertebral height.
Table 1.
Mimics of spinal tuberculosis.
| Mimics of spinal tuberculosis | Salient features to differentiate it from spinal tuberculosis. |
|---|---|
| Pyogenic spondylitis (Fig. 13) | Acute onset with shorter duration of history. High fever and constitutional symptoms. Lumbar spine is the most common site. Usually involve ≤2 vertebral bodies. More homogeneous and ill-defined enhancement of involved vertebral bodies <50% vertebral body destruction. Early and severe disc involvement. Paraspinal abscess are infrequent and when present show thick and irregular wall enhancement.19,24 Functional MRI, particularly diffusion tensor imaging, and dynamic contrast enhanced MRI, are new emerging techniques used to differentiate pyogenic spondylitis from spinal tuberculosis.26,27 Razek, and Sherif noted higher mean diffusivity, and lower fractional anisotropy in pyogenic spondylitis, than in spinal tuberculosis.26 Miyamoto and Akagi noted longer maximum contrast index, and higher likelihood of enhanced disc in pyogenic spondylitis, in comparison with spinal tuberculosis.27 |
| Brucellosis | Predilection for lumbar spine. Diffuse vertebral osteomyelitis with preserved vertebral architecture. Gibbus deformity is rare. Osteophyte formation at anterior vertebral end plate (parrot's beak) Facet joint involvement and early disc involvement. Air with in intervertebral disc or vertebral body is characteristic finding in brucellosis. Paraspinal abscess, when present, show thin irregular wall enhancement.24,25,28 |
| Andersson's lesion in ankylosing spondylitis | Localized disco-vertebral inflammation or fracture of ankylosed spine. Associated features of ankylosing spondylitis: Syndesmophytes, bamboo spine, facet arthropathy, and bilateral sacroiliitis.24,25 |
| SAPHO (Synovitis, Acne, Pustulosis, Hyperostosis, Osteitis) syndrome | Multifocal involvement. Anterior vertebral body corner erosions are characteristic finding. Absence of abscess. Extraspinal involvement, particularly skin manifestations are often seen (94%).25,29 |
| Rheumatoid arthritis | Cervical spine is most common site of spinal involvement. Almost always associated with other features of rheumatoid arthritis, more commonly, peripheral polyarthropathy. Absence of abscess.24,30 |
| Modic type 1 changes | Lack of clinical features and laboratory findings suggestive of infective etiology, such as fever, and raised white blood cell counts or ESR. Absence of abnormal high signal intensity with in disc. End plate erosion rather than end plate destruction. Absence of paraspinal abscess. Vacuum phenomenon.24,25,31 |
| Baastrup's disease | Commonly involve L4-L5 spinous process. Approximation of enlarged spinous process. Often associated with degenerative spine changes as loss of disc height, osteophyte formation, spondylosis, and spondylolisthesis.24 |
| Osteoporotic fracture | T2 and T1 hypointense fracture line or trabecular impaction. Relatively preserved marrow signal intensity. Absent paraspinal soft tissue or abscess. Diffuse osteoporotic changes.24 |
| Neuropathic spine | Appropriate clinical history is important, particularly of traumatic spinal cord injury or neurosyphilis. Gas with in the disc, bone sclerosis, large osteophytes, bone fragmentation, and malalignment.24,25 |
| Metastasis (Fig. 14) | History of malignancy. Thoracic spine is the most common site involved. Single vertebral body or posterior elements involvement favor metastasis. Presence of skip lesion. Destructive bone lesion with preserved disc and sharp endplates. No sequestra formation. Absence of paraspinal or intra-osseus abscess.24,32,33 |
Among above mentioned conditions, differentiation of spinal metastasis from spinal tuberculosis is of great clinical significance.34 Spinal tuberculosis is a benign disease, and is managed by effective antitubercular therapy,10 whereas, spinal metastasis is a malignant disease, and surgery or chemotherapy may be optimal therapy.35 Although, biopsy is gold standard to distinguish spinal tuberculosis from spinal metastasis,36 diagnosis at outpatient department is often based on clinical findings, and imaging.33 Important features that help differentiate spinal metastasis from spinal tuberculosis include (i) history of malignant tumor, (ii) posterior element destruction, (iii) preserved intervertebral disc, (iv) absence of sequestrum, and (v) absence subligamentous spread33,34 (Fig. 14). Du et al. developed a scoring system (Table 2) based on above five clinical characteristics, with total score ranging from 0 to 10. Higher scores were noted in spinal metastasis, than in spinal tuberculosis. Score 5 has been suggested as optimal cut-off to differentiate spinal metastasis from spinal tuberculosis, with area under curve of 96.5%.33
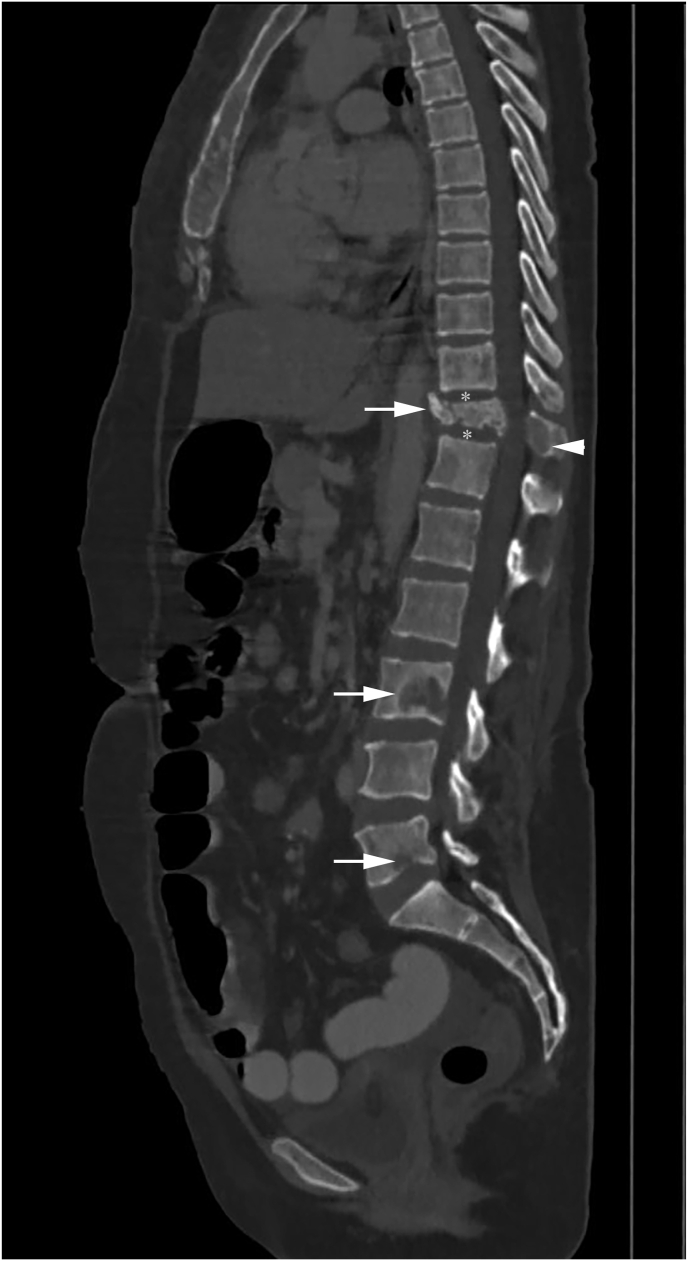
Fig. 14.
Spinal metastasis in a patient with infiltrating ductal carcinoma of the breast. Sagittal CT bone window image shows lytic lesions involving multiple non-contiguous vertebrae (skip lesions) (white arrow) with metastatic compression fracture of the D11 vertebra body, and involvement of posterior elements (white arrowhead). Intervertebral disc spaces are maintained (white asterisk).
Table 2.
Scoring system to differentiate spinal metastasis from spinal tuberculosis by Du et al.33
| Clinical/Radiological characteristics | Yes | No |
| History of malignancy | 2 | 0 |
| Destroyed vertebral posterior elements | 2 | 0 |
| Preserved intervertebral disc | 3 | 0 |
| No sequestra formation | 2 | 0 |
| Absence of subligamentous spread | 1 | 0 |
Diffusion weighted imaging, and dynamic contrast enhanced (DCE) MRI are also evaluated to differentiate spinal tuberculosis from spinal metastasis. Few studies have shown lower apparent diffusion coefficient (ADC) in metastatic vertebrae than in spinal tuberculosis. However, there was overlap between ADC values of metastatic vertebrae and spinal tuberculosis in some patients, suggesting that diffusion images and ADC values should be interpreted in the background of clinical history and routine MRI findings.37, 38, 39 Lang et al. noted that kinetic time course of DCE MRI is more likely to show washout/plateau pattern in metastatic vertebrae, whereas, washout pattern is less likely in spinal tuberculosis. Washout rate constant, Kep, was seen to be significantly lower in spinal tuberculosis than in spinal metastasis.40
7. Imaging interventions in spinal tuberculosis
7.1. Percutaneous vertebral biopsy
CT is the best imaging modality to guide percutaneous vertebral biopsy for tissue sampling and confirmation of etiology.13 Over open biopsy, CT guided biopsy is advantageous, that it is quick, easy, accurate, safe and has less procedure related complications. Procedure is usually performed under local anesthesia. Posterior entry is preferred in majority of the cases, except in lower cervical spine where anterolateral entry is preferred. Common approaches in CT guided percutaneous vertebral biopsy include transpedicular approach, translaminar approach, trans-costovertebral approach, and trans-apophyseal approach. Preferably two types of samples are obtained. First, fine needle aspiration biopsy which contains block of cells for microbiological evaluation, and second, large bore biopsy using 8G to17G needle, for histopathological evaluation. Genotypic analysis, and immunohistochemistry can be done on both type of samples. Biopsy is more effective if taken from lytic areas rather than sclerotic areas, and also from solid areas than liquified component. However, aspiration can be done in case of liquefied abscess.13
7.2. Percutaneous drainage of cold abscess
Percutaneous drainage can be done using both ultrasound or CT guidance. It is safe and effective alternative to open surgical drainage with less procedure related morbidity. It is indicated when there are associated neurological pressure effects, or when there is poor response to medical treatment. It can also be done when there is large iliopsoas abscess, to aid the medical treatment (13).
8. Imaging evidence of healing
WHO recommends category I antitubercular therapy for 9 months in newly diagnosed spinal tuberculosis.5 Fixed time schedule in treatment of spinal tuberculosis is considered unscientific and many experts advise longer duration of therapy guided by either pathological, clinical, or imaging evidence of response. Though repeat tissue sampling at the end of treatment regimen is the best method of documenting healing, it is invasive, and not practical in spinal tuberculosis. Monitoring of therapeutic response is often based on clinical evaluation and imaging. Neuroimaging, particularly MRI is commonly used in evaluation of treatment response. It is advised to obtain MRI at 8months of therapy and subsequently, as required, to look for MR signs of healing. Currently, MRI is still imperfect in documenting healed status. Better criteria of healing than MRI is needed and 18F-FDG PET can be one such modality.4,5
8.1. Radiographic signs of healing
Radiological signs of healing often appear quite late on plain radiograph. On radiograph, bone destruction and loss of vertebral height can occur even during therapy, and shouldn't not be taken as sign of poor therapeutic response.41 Common signs of healing that are described on radiograph6,13,41 include:
-
(i)
Sclerosis is a sign of healing when early disease is purely osteolytic. It should be seen with in 5 months of treatment, and can progress over a period of 1 year. However, sclerosis, when present prior to antitubercular therapy, is of little importance in monitoring response;
-
(ii)
Paravertebral soft tissue densities, if seen on initial radiograph, is useful feature on follow up. Paravertebral masses often reach maximum size with in one and half months of presentation. Decrease in these soft tissue densities is a sign of healing. However, they could sometimes take up to 15 months to completely resolve.
-
(iii)
Sharpening of articular and cortical margins.
-
(iv)
Remineralization and reformation of bony trabeculae.
-
(v)
Bony ankylosis is believed to be sure sign of healing, and is seen in around 50% of patients.6,13,41
8.2. Signs of healing on MRI
MRI is the most common imaging modality used to monitor therapeutic response. Even though MRI is highly sensitive in diagnosis of spinal tuberculosis, assessment of healing on MRI can be difficult.13 Common signs of healing on MRI (Fig. 15) include.
-
(i)
Resolution of bone marrow inflammatory edema is seen with healing. However, bone marrow edema can sometimes persist for up to 14 months, and it might be difficult to differentiate active infection from sterile residual abnormality.6,7
-
(ii)
Decrease in contrast enhancement is early sign of healing, and can be seen within few weeks or months of treatment. However, in around 15% of patients, contrast enhancement can persist even after successful treatment.13
-
(iii)
Decrease in paravertebral, and epidural abscess and granulation tissue can be seen with healing. Small collections with thin rim enhancement may persist and represent sterile collections.6,7,13
-
(iv)
Fatty change with in the bone marrow seen as hyperintense signal on both T1, and T2 weighted images, matching that of fat, is seen with healing. However, it is a gradual process, and is seen in 40% of patients at 6 months and 75% of patients at 12 months of treatment.6,7,13
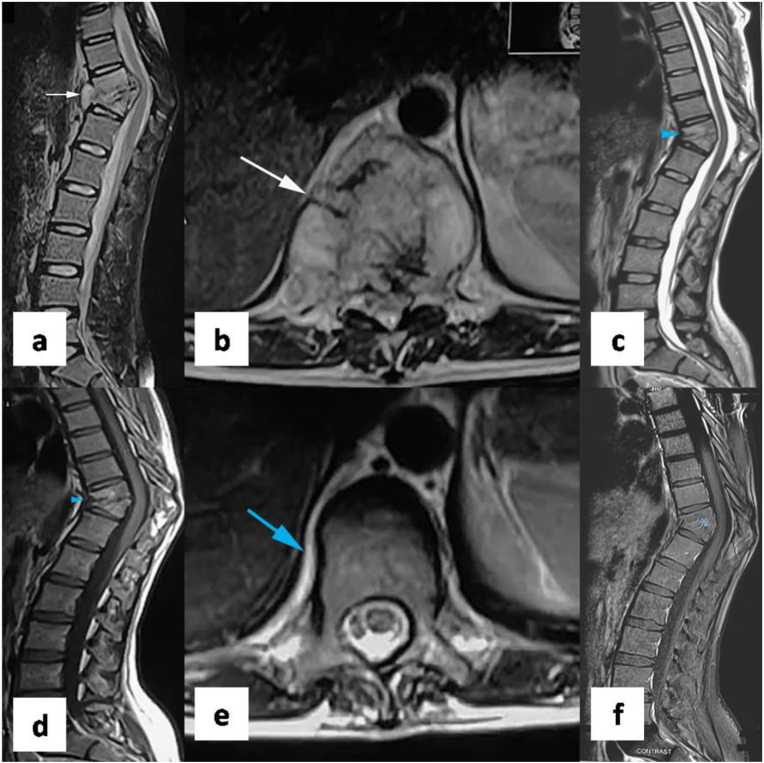
Fig. 15.
MRI features of healing. (a) sagittal STIR and (b) axial T2 weighted images show paradiscal involvement of D10-D11 vertebrae with wedge collapse of D10 and D11 vertebral bodies, inflammatory bone marrow edema, and paravertebral soft tissue (white arrow) consistent with active spinal tuberculosis. Follow up MRI was done after 8 months of antitubercular therapy. (c) Sagittal T2 weighted and (d) sagittal T1 weighted images of follow up MRI shows bony ankylosis with fatty change in bone marrow (blue arrowhead). (e) axial T2 weighted image of follow up MRI shows resolution of paravertebral soft tissue (blue arrow). (f) Sagittal T1 weighted post contrast images of follow up MRI shows no abnormal contrast enhancement (blue asterisk). Follow up MRI features are consistent with healing.
8.3. 18F-FDG PET in evaluation of healing
18F-FDG PET evaluates metabolic activity in the lesion in a non-invasive, and semiquantitative manner. Active tubercular lesions show presence of lymphocytes, epitheloid cells, and Langerhans type of giant cells, which show high glucose utilisation and thus, high FDG uptake.7,42 Decrease in FDG uptake can indicate response to anti-tubercular therapy and guide duration of treatment.7,43,44 It is also particularly useful in evaluation of therapeutic response in presence of metallic implants, where MRI is of limited use.13 Simultaneous 18F-FDG PET/MRI has both metabolic advantage of PET and anatomical advantage of MRI, for evaluation of treatment response in spinal tuberculosis, and can be used to determine appropriate time to discontinue anti-tubercular treatment.7 Further, 18F-FDG PET may be useful in early detection of non-responders, and multi drug resistant tuberculosis (MDR-TB), reducing the lag in the management of MDR-TB and its associated morbidity.45,46
9. Conclusion
Spinal tuberculosis is a common extrapulmonary tuberculosis with potential to cause serious complications such as spinal deformity, and neurological disability. Early diagnosis and timely management are necessary to prevent disabling complications of spinal tuberculosis. Imaging, particularly MRI, is useful in early diagnosis of spinal tuberculosis. Imaging features such as paradiscal involvement, >50% vertebral destruction, Subligamentous spread of infection to three or more contiguous vertebral levels, large abscess with smooth thin wall, and calcification with in paravertebral soft tissue, are useful in differentiating spinal tuberculosis from its mimics. Imaging, particularly MRI, and 18F-FDG PET, play an important role in evaluation of therapeutic response, and help to decide when to stop anti tubercular therapy.
Scope of the manuscript
The manuscript aims to consolidate the existing knowledge of imaging in spinal tuberculosis, essential for timely diagnosis and treatment.
Description of any subject overlap with previously published works
None.
Confirmation of sole submission to the Journal of Clinical Orthopaedics and Trauma
We confirm that the above-mentioned review article titled ‘Imaging update in spinal tuberculosis’ has been solely submitted to the ‘Journal of Clinical Orthopaedics and Trauma’.
Declaration of competing interest
None.
Footnotes
All the authors have significantly contributed to literature review, data collection, manuscript writing, and proof reading.
Contributor Information
Vijay Kubihal, Email: vijaysk91@gmail.com.
Raju Sharma, Email: drrajuaiims@gmail.com.
R.G. Krishna Kumar, Email: krishna.aiimsrad@gmail.com.
S.H. Chandrashekhara, Email: drchandruaiims@yahoo.com.
Rakesh Garg, Email: drrgarg@hotmail.com.
References
- 1.Duraiswami P.K., Orth M., Tuli S.M. 5000 years of orthopaedics in India. Clin Orthop. 1971 Apr;75:269–280. PMID: 4929008. [PubMed] [Google Scholar]
- 2.Rajasekaran S., Soundararajan D.C.R., Shetty A.P., Kanna R.M. Spinal tuberculosis: current concepts. Global Spine J. 2018 Dec;8(4_suppl):96S–108S. doi: 10.1177/2192568218769053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuli S.M. Historical aspects of Pott's disease (spinal tuberculosis) management. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2013 Jun;22(Suppl 4):529–538. doi: 10.1007/s00586-012-2388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain A.K. Tuberculosis of spine: research evidence to treatment guidelines. Indian J Orthop. 2016 Feb;50(1):3–9. doi: 10.4103/0019-5413.173518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg D., Goyal V. Spinal tuberculosis treatment: an enduring bone of contention. Ann Indian Acad Neurol. 2020;23(4):441. doi: 10.4103/aian.AIAN_141_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh R., Magu N.K., Rohilla R.K. Clinicoradiologic profile of involvement and healing in tuberculosis of the spine. Ann Med Health Sci Res. 2016 Oct;6(5):311–327. doi: 10.4103/amhsr.amhsr_188_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeon I., Kong E., Kim S.W. Simultaneous 18F-FDG PET/MRI in tuberculous spondylitis: an independent method for assessing therapeutic response - case series. BMC Infect Dis. 2019 Oct 15;19(1):845. doi: 10.1186/s12879-019-4469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moorthy S., Prabhu N.K. Spectrum of MR imaging findings in spinal tuberculosis. Am J Roentgenol. 2002 Oct;179(4):979–983. doi: 10.2214/ajr.179.4.1790979. [DOI] [PubMed] [Google Scholar]
- 9.Khanna K., Sabharwal S. Spinal tuberculosis: a comprehensive review for the modern spine surgeon. Spine J. 2019 Nov;19(11):1858–1870. doi: 10.1016/j.spinee.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Dunn R.N., Ben Husien M. Spinal tuberculosis: review of current management. Bone Jt J. 2018 Apr 1;100-B(4):425–431. doi: 10.1302/0301-620X.100B4.BJJ-2017-1040.R1. [DOI] [PubMed] [Google Scholar]
- 11.Hasan Khan M.N., Jamal A.B., Hafeez A., Sadiq M., Rasool M.U. Is spinal tuberculosis changing with changing time? Ann Med Surg. 2021 Jun;66:102421. doi: 10.1016/j.amsu.2021.102421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garg R.K., Somvanshi D.S. Spinal tuberculosis: a review. J Spinal Cord Med. 2011 Sep;34(5):440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivas-Garcia A., Sarria-Estrada S., Torrents-Odin C., Casas-Gomila L., Franquet E. Imaging findings of Pott's disease. Eur Spine J. 2013 Jun;22(S4):567–578. doi: 10.1007/s00586-012-2333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pohlan J. Pyogenic spondylodiscitis: dual-energy computed tomography reveals disc destruction in an 80-year-old patient. 2020 Mar 3. https://www.eurorad.org/case/16622 [cited 2021 Apr 11]; Available from: [DOI]
- 15.Yuan Y., Lang N., Yuan H. Rapid-kilovoltage-switching dual-energy computed tomography (CT) for differentiating spinal osteolytic metastases from spinal infections. Quant Imag Med Surg. 2021 Feb;11(2):620–627. doi: 10.21037/qims-20-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Shi S., Zheng Q., Jin Y., Dai Y. Application of 3-dimensional printing technology combined with guide plates for thoracic spinal tuberculosis. Medicine (Baltim) 2021 Feb 12;100(6) doi: 10.1097/MD.0000000000024636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagashima H., Tanishima S., Tanida A. Diagnosis and management of spinal infections. J Orthop Sci Off J Jpn Orthop Assoc. 2018 Jan;23(1):8–13. doi: 10.1016/j.jos.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Lacerda C., Linhas R., Duarte R. Tuberculous spondylitis: a report of different clinical scenarios and literature update. Case Rep Med. 2017. 2017:1–4. doi: 10.1155/2017/4165301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee K.Y. Comparison of pyogenic spondylitis and tuberculous spondylitis. Asian Spine J. 2014 Apr;8(2):216–223. doi: 10.4184/asj.2014.8.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanna R.M., Babu N., Kannan M., Shetty A.P., Rajasekaran S. Diagnostic accuracy of whole spine magnetic resonance imaging in spinal tuberculosis validated through tissue studies. Eur Spine J. 2019 Dec;28(12):3003–3010. doi: 10.1007/s00586-019-06031-z. [DOI] [PubMed] [Google Scholar]
- 21.Priftakis D., Riaz S., Zumla A., Bomanji J. Towards more accurate 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) imaging in active and latent tuberculosis. Int J Infect Dis. 2020 Mar;92:S85–S90. doi: 10.1016/j.ijid.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 22.Gnanasegaran G., Barwick T., Milburn H., Vijayanathan S., Fogelman I. Tuberculosis of the spine on Tc-99m MDP bone scan: additional role of SPECT-CT. Clin Nucl Med. 2009 May;34(5):271–274. doi: 10.1097/RLU.0b013e31819e52e8. [DOI] [PubMed] [Google Scholar]
- 23.Kumar K. Spinal tuberculosis, natural history of disease, classifications and principles of management with historical perspective. Eur J Orthop Surg Traumatol Orthop Traumatol. 2016 Aug;26(6):551–558. doi: 10.1007/s00590-016-1811-x. [DOI] [PubMed] [Google Scholar]
- 24.Kumaran S., Thippeswamy P., Reddy B., Neelakantan S., Viswamitra S. An institutional review of tuberculosis spine mimics on MR imaging: cases of mistaken identity. Neurol India. 2019;67(6):1408. doi: 10.4103/0028-3886.273630. [DOI] [PubMed] [Google Scholar]
- 25.Hong S.H., Choi J.-Y., Lee J.W., Kim N.R., Choi J.-A., Kang H.S. MR imaging assessment of the spine: infection or an imitation? Radiographics. 2009 Mar;29(2):599–612. doi: 10.1148/rg.292085137. [DOI] [PubMed] [Google Scholar]
- 26.Abdel Razek A.A.K., Mohamed Sherif F. Assessment of diffusion tensor imaging in differentiation between pyogenic and tuberculous spondylitis. Eur J Radiol. 2021 Jun;139:109695. doi: 10.1016/j.ejrad.2021.109695. [DOI] [PubMed] [Google Scholar]
- 27.Miyamoto H., Akagi M. Usefulness of dynamic contrast-enhanced magnetic resonance images for distinguishing between pyogenic spondylitis and tuberculous spondylitis. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2019 Dec;28(12):3011–3017. doi: 10.1007/s00586-019-06057-3. [DOI] [PubMed] [Google Scholar]
- 28.Li T., Liu T., Jiang Z., Cui X., Sun J. Diagnosing pyogenic, brucella and tuberculous spondylitis using histopathology and MRI: a retrospective study. Exp Ther Med. 2016 Oct;12(4):2069–2077. doi: 10.3892/etm.2016.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGauvran A.M., Kotsenas A.L., Diehn F.E., Wald J.T., Carr C.M., Morris J.M. SAPHO syndrome: imaging findings of vertebral involvement. Am J Neuroradiol. 2016 Aug 1;37(8):1567–1572. doi: 10.3174/ajnr.A4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jurik A.G. Imaging the spine in arthritis-a pictorial review. Insights Imaging. 2011 Apr;2(2):177–191. doi: 10.1007/s13244-010-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon J.W., Yoon Y.C., Choi S.-H., Jung J.Y., Choe B.K. MR imaging for the differentiation of early infectious spondylitis and modic type I change in the lumbar spine. J Korean Soc Radiol. 2010;62(6):563. doi: 10.3348/JKSR.2010.62.6.563. [DOI] [Google Scholar]
- 32.Mittal S., Khalid M., Sabir A.B., Khalid S. Comparison of magnetic resonance imaging findings between pathologically proven cases of atypical tubercular spine and tumour metastasis: a retrospective study in 40 patients. Asian Spine J. 2016 Aug;10(4):734–743. doi: 10.4184/asj.2016.10.4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du X., She Y., Ou Y., Zhu Y., Luo W., Jiang D. A scoring system for outpatient orthopedist to preliminarily distinguish spinal metastasis from spinal tuberculosis: a retrospective analysis of 141 patients. Dis Markers. 2021. 2021:6640254. doi: 10.1155/2021/6640254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan D., Saddique M.U., Paul T., Murshed K., Zahid M. Metastatic adenocarcinoma of the lung mimicking miliary tuberculosis and pott's disease. Cureus [internet] 2021 Jan 22. https://www.cureus.com/articles/43423-metastatic-adenocarcinoma-of-the-lung-mimicking-miliary-tuberculosis-and-potts-disease [cited 2021 Aug 15]; Available from: [DOI] [PMC free article] [PubMed]
- 35.Harel R., Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer. 2010 Oct;46(15):2696–2707. doi: 10.1016/j.ejca.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Rehm J., Veith S., Akbar M., Kauczor H., Weber M. CT-guided percutaneous spine biopsy in suspected infection or malignancy: a study of 214 patients. RöFo - Fortschritte Auf Dem Geb Röntgenstrahlen Bildgeb Verfahr. 2016 Dec 1;188(12):1156–1162. doi: 10.1055/s-0042-116233. [DOI] [PubMed] [Google Scholar]
- 37.Palle L., Reddy M.B., Reddy K.J. Role of magnetic resonance diffusion imaging and apparent diffusion coefficient values in the evaluation of spinal tuberculosis in Indian patients. Indian J Radiol Imag. 2010 Nov;20(4):279–283. doi: 10.4103/0971-3026.73544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madhok R., Sachdeva P. Evaluation of apparent diffusion coefficient values in spinal tuberculosis by MRI. J Clin Diagn Res JCDR. 2016 Aug;10(8):TC19–23. doi: 10.7860/JCDR/2016/20520.8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abo Dewan K.A.W., Salama A.A., El habashy H.M.S., Khalil A.E.S. Evaluation of benign and malignant vertebral lesions with diffusion weighted magnetic resonance imaging and apparent diffusion coefficient measurements. Egypt J Radiol Nucl Med. 2015 Jun;46(2):423–433. doi: 10.1016/j.ejrnm.2015.01.002. [DOI] [Google Scholar]
- 40.Lang N., Su M.-Y., Yu H.J., Yuan H. Differentiation of tuberculosis and metastatic cancer in the spine using dynamic contrast-enhanced MRI. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015 Aug;24(8):1729–1737. doi: 10.1007/s00586-015-3851-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boxer D.I., Pratt C., Hine A.L., McNicol M. Radiological features during and following treatment of spinal tuberculosis. Br J Radiol. 1992 Jun;65(774):476–479. doi: 10.1259/0007-1285-65-774-476. [DOI] [PubMed] [Google Scholar]
- 42.Metser U., Even-Sapir E. Increased (18)F-fluorodeoxyglucose uptake in benign, nonphysiologic lesions found on whole-body positron emission tomography/computed tomography (PET/CT): accumulated data from four years of experience with PET/CT. Semin Nucl Med. 2007 May;37(3):206–222. doi: 10.1053/j.semnuclmed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Park I.-N., Ryu J.-S., Shim T.S. Evaluation of therapeutic response of tuberculoma using F-18 FDG positron emission tomography. Clin Nucl Med. 2008 Jan;33(1):1–3. doi: 10.1097/RLU.0b013e31815c5128. [DOI] [PubMed] [Google Scholar]
- 44.Hofmeyr A., Lau W.F.E., Slavin M.A. Mycobacterium tuberculosis infection in patients with cancer, the role of 18-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring treatment response. Tuberc Edinb Scotl. 2007 Sep;87(5):459–463. doi: 10.1016/j.tube.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Dureja S., Sen I.B., Acharya S. Potential role of F18 FDG PET-CT as an imaging biomarker for the noninvasive evaluation in uncomplicated skeletal tuberculosis: a prospective clinical observational study. Eur Spine J. 2014 Nov;23(11):2449–2454. doi: 10.1007/s00586-014-3483-8. [DOI] [PubMed] [Google Scholar]
- 46.Bomanji J., Sharma R., Mittal B.R., et al. Sequential 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) scan findings in patients with extrapulmonary tuberculosis during the course of treatment—a prospective observational study. Eur J Nucl Med Mol Imag. 2020 Dec;47(13):3118–3129. doi: 10.1007/s00259-020-04888-7. [DOI] [PubMed] [Google Scholar]