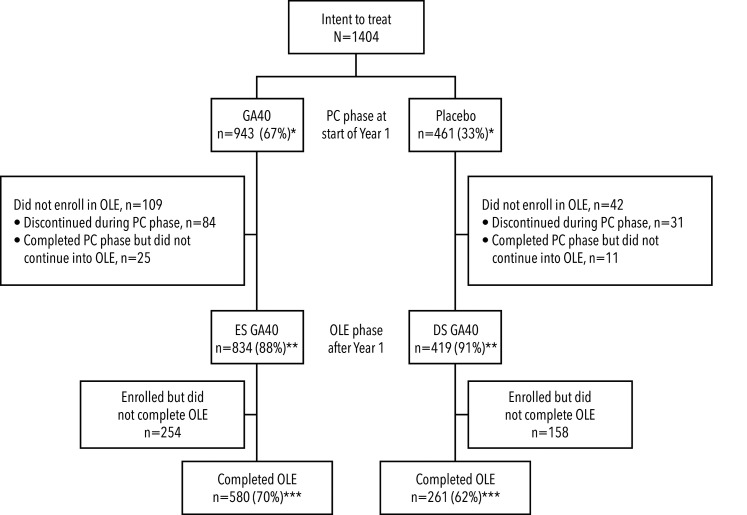
Figure 1.
Patient disposition
*as percentage of intent-to-treat patients; **as percentage of patients in PC phase at start of Year 1; ***as percentage of patients in OLE phase after Year 1.
DS: delayed start; ES: early start; GA40: glatiramer acetate 40 mg/mL subcutaneous injection administered three times weekly; OLE: open-label extension; PC: placebo-controlled.