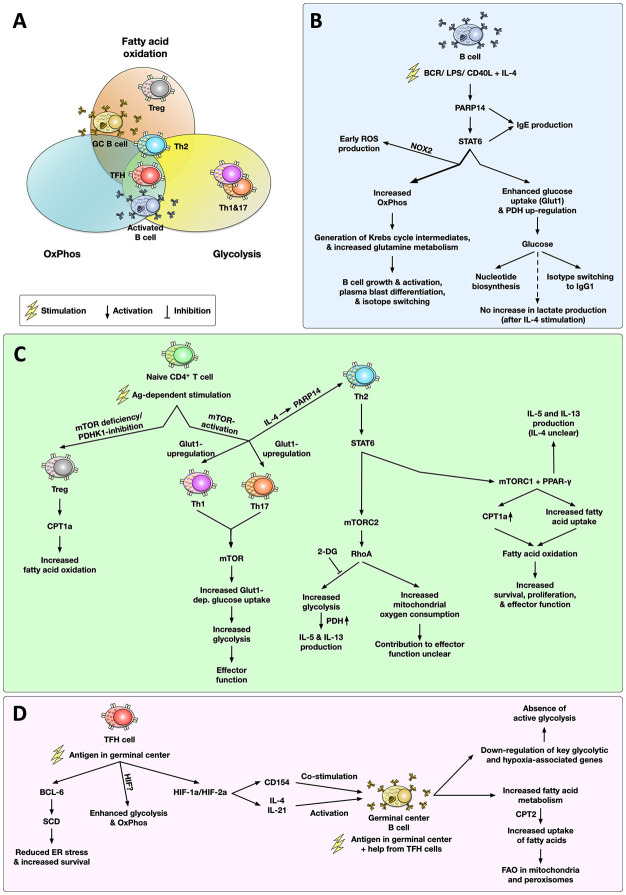
Figure 2.
Metabolic phenotype and main signaling pathways associated with the activation of T and B cells in allergies. Cell types are grouped within the metabolic pathways (glycolysis, oxidative phosphorylation (OxPhos), fatty acid oxidation (FAO)) according to the published and discussed literature (A). Upon activation, IL-4-stimulated B cells undergo complex metabolic changes, including a poly ADP-ribose polymerase 14 (PARP14)-dependent increase in glucose consumption driving both nucleotide synthesis and IgG1 production as well as high rates of OxPhos and glutamine metabolism, which promote B cell activation, plasmablast differentiation, and isotype switching (B). In naïve CD4+ T cells antigen-dependent stimulation results in a mechanistic target of rapamycin (mTOR)-dependent T helper cell differentiation. While inhibition of mTOR results in differentiation of regulatory T cells (Treg) with a predominant carnitine palmitoyl transferase 1a (CPT1a)-dependent increase in FAO, mTOR activation is critically important for either Th1, Th2, Th17 differentiation. In both Th1 and Th17 cells, mTOR activation drives a glycolytic phenotype. In Th2 cells, mTOR complex 2 (mTORC2) promotes a RhoA-dependent increase in glycolysis (which was shown to contribute to IL-5 and IL-13 production) and an increase in mitochondrial oxygen consumption (whose contribution to Th2 effector function is currently unclear). Moreover, activation of mTORC1 and peroxisome proliferator-activated receptor gamma (PPAR-γ) in Th2 cells promotes fatty acid uptake and oxidation which fuels Th2 cell survival, proliferation, and effector function (C). In germinal centers (GC), follicular T helper cells (TFH) HIF-1/2a-dependently promote the activation of germinal center B cells via CD154-dependent co-stimulation and the production of the switching cytokines IL-4 and IL-21 (D). Here, TFH cells display both enhanced glycolysis and OxPhos while using the lipid metabolism enzyme stearoyl-CoA desaturase (SCD) to reduce ER stress and increase survival. In contrast to other cells types, antigen-activated GC B cells mainly rely on FAO for energy generation while suppressing glycolytic genes. For more detailed information see text. OxPhos, oxidative phosphorylation; FAO, fatty acid oxidation; BCR, B cell receptor; LPS, lipopolysaccharide; Glut1, glucose transporter 1; TCR, T cell receptor; 2-DG, 2-deoxy glucose; mTOR(C1/2), mechanistic target of rapamycin complex 1/2; PDH(K1), pyruvate dehydrogenase (kinase 1); LDH, lactate dehydrogenase; Treg, regulatory T cell; CPT1a/2, carnitine palmitoyl transferase 1a/2; PARP14, Poly ADP-ribose polymerase 14; PPAR-γ, peroxisome proliferator-activated receptor gamma; ROS, reactive oxygen species; STAT6, signal transducer and activator of transcription 6; NOX2, NADPH oxidase 2; BCL-6, B-cell lymphoma 6 protein; GC, germinal center; TFH cell, follicular T helper cell; SCD, stearoyl-CoA desaturase; HIF-1/2a, hypoxia inducible factor 1/2a.