Table 3.
Prodrug of Bisphosphonate for Tumor Treatment
| Prodrug | Structure | Efficacy |
|---|---|---|
| 12b80 compound82 | 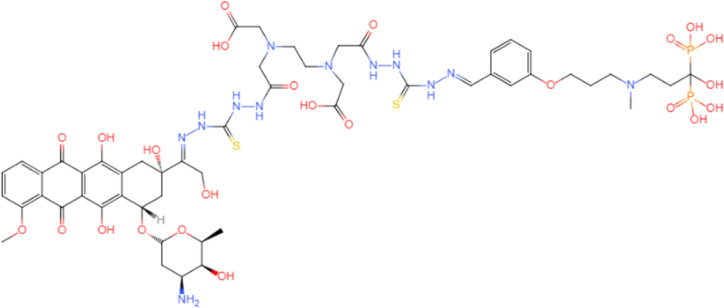 |
High affinity of bone support, specific release of doxorubicin, low cytotoxicity and cell uptake of prodrugs. |
| Bisphosphora–midate prodrug83 |  |
Significantly enhanced anti-cancer activity |
| Tetrakis-pivaloyloxy–methyl 2-(thiazole-2-ylamino) ethylidene-1, 1- bisphosphonate (7)84 |  |
Improve the effectiveness of related cancer immunotherapy |
| Prodrug micelles (alendronic acid - nanoparticle)85 |  |
Reduce systemic toxicity, improve therapeutic effect |
| Doxorubicin bisphosphonate prodrug86 |  |
Good stability and high affinity |
| 2-(thiazole-2-ylamino) ethylidene-1, 1-bisphosphonate87 | 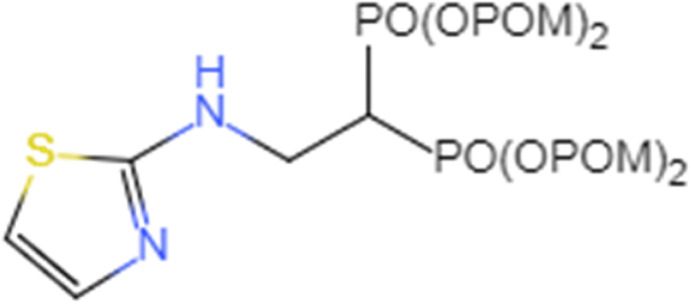 |
Directly act on tumor growth, expand cytotoxic Vg2Vd2T cells in vitro, and enhance tumor control |
| Fluorine—containing zoledronate prodrug88 |  |
Sensitize tumor cells for killing, expand Vγ2Vδ2 T cells for adoptive cell therapy |
