Abstract
We report a 41-year-old man diagnosed with the adult form of hypophosphatasia (HPP) and treated for 4 years with less frequent than conventional daily doses of teriparatide (TPTD). He presented with a history of three low-energy fractures and low bone mineral density (BMD) ineffectively treated with bisphosphonate. We identified within ALPL, the gene that encodes the homodimeric “tissue-nonspecific” isoenzyme of alkaline phosphatase (ALP) and underlies HPP, a heterozygous missense mutation (c.455 G>A→R135H). Characteristic painful periarticular calcification removed at a shoulder did not recur. However, access to medical treatment with asfotase alfa (AA) was denied. After he sustained a low-energy metatarsal fracture, we administered TPTD subcutaneously “off-label” at 20 µg/d. An elbow fracture occurred two months later. Five months afterwards, due to his limited number of approved TPTD doses, TPTD treatment was extended using alternate-day dosing. Although his serum ALP activity did not increase (33-48 U/l; reference range 40-120) with 4 years of TPTD treatment, his BMD improved 15% in the lumbar spine and 6% in the femoral neck with no further fractures. Our experience represents success overcoming two prescription deadlocks; AA was denied for adult HPP, and TPTD was not to be administered daily for more than two years.
Keywords: Alkaline Phosphatase, Asfotase Alfa, Fracture, Hypophosphatasia, Teriparatide
Introduction
Hypophosphatasia (HPP) is the heritable dento-osseous disease characterized in the clinical laboratory foremost by low serum alkaline phosphatase (ALP) activity (hypophosphatasemia)[1,2]. This biochemical hallmark reflects loss of function mutation(s) within the ALPL gene, which encodes the homodimeric “tissue non-specific” isoenzyme of ALP (TNSALP)[3]. More than 400 mutations, commonly missense, causing HPP have been identified in ALPL (http://www.sesep.uvsq.fr/03_hypo_mutations.php), which largely explains the remarkably broad-ranging severity of this inborn-error-of-metabolism[2,4]. The pathogenesis of HPP involves extracellular accumulation of the TNSALP substrates inorganic pyrophosphate (PPi), an inhibitor of mineralization, and pyridoxal 5,-phosphate (PLP), the principal circulating vitameric form of vitamin B6[2-3].
HPP is classified clinically according to severity, i.e., perinatal (the typically lethal form), infantile, mild or severe childhood, adult, and odonto-HPP (the most mild form, compromising only the teeth)[2,3]. Adult HPP usually presents during middle-age with recurrent, low-energy, appendicular fractures (e.g., metatarsal stress fractures) that eventually fail to heal, as well as with pseudofractures, which are a radiological hallmark of osteomalacia[2]. The dentition may also be defective[1,2]. Bone mineral density (BMD) can be low, normal, or high in adult HPP[5]. Calcific periarthritis may occur, featuring deposition of hydroxyapatite around major joints[6], and chondrocalcinosis is often prominent. Thus, adult HPP compromises the quality of life, and may be debilitating owing to recurrent fracturing of the limbs accompanied by musculoskeletal and joint pain[7].
Asfotase alfa (AA), a recombinant hydroxyapatite-targeted TNSALP-replacement therapy, is effective for HPP at all ages[8]. Although approved for life-threatening or debilitating pediatric-onset HPP, its high cost seems to underlie why this biologic is often not endorsed for adult-onset HPP, apart from Japan where it is available when necessary for all patients[2]. Hence, teriparatide (TPTD), recombinant parathyroid hormone (PTH) 1-34[9], has been administered “off label” to some adults with HPP, prompting their osteoblasts to synthesize more TNSALP[10-12]. Reportedly, this has helped some, but not all[13], such patients by alleviating bone pain and healing “stress” and pseudofractures[10]. Perhaps TPTD treatment for adult HPP is more successful when there is ALPL mutation heterozygosity, i.e., the presence of one normal ALPL allele that can be transcribed to synthesize additional intact homodimeric TNSALP[2].
We report a middle-aged man with adult HPP who apparently benefitted from 4 years of TPTD treatment, unconventionally administered less often than daily.
Case presentation
At presentation to us (February 2016), this 41-year-old Greek man [height 187 cm (6 ft, 2 in), weight 106 kg (234 lbs), body mass index (BMI) 30.3 kg/m2] reported three low-energy fractures during 2014-2015 (right elbow twice, and right third metatarsal). Each had been managed conservatively without subsequent pharmacologic treatment, and before we diagnosed his HPP. There had been no prior fractures. He gave no history of early loss of primary teeth or rickets. He had flatfeet, chronic headache, and mild musculoskeletal pain (since 2010), for which he received pregabalin (75 mg po twice daily). Arterial hypertension and dyslipidemia (with high lipoprotein(a) 149 mg/dl; normal <30) was treated with metoprolol (75 mg po/d) and rosuvastatin (10 mg po/d). Calcinosis at his right shoulder (Figure 1A), a feature of adult HPP[6], had been partially removed by surgery (December 2015) with symptom improvement. Histology of the lesion had not been performed. Two months after surgery, there was less calcinosis (Figure 1B). Despite his tall stature, in July 2015 lumbar spine bone mineral density (BMD) (Lunar Prodigy, GE Healthcare, Chicago, IL) was reported to be 0.992 g/cm2 (Z-score -2.8) and femoral neck BMD 0.726 g/cm2 (Z-score -2.8). Based on these BMD values, he had commenced treatment (September 2015) elsewhere with a weekly combination of alendronate (70 mg) and cholecalciferol (5600 IU) administered orally. This was stopped at presentation to us (February 2016), because we noted that low or low-normal serum ALP activity had been documented during all of his past routine check-ups (33-45 U/l; reference range 40-120), including long before the bisphosphonate treatment[14]. His serum calcium was high-normal (10.2 mg/dl; reference range 8.5-10.5); phosphate, albumin, and PTH normal; and 25-hydroxyvitamin D [25(OH)D] sufficient (33.7 ng/ml). Subsequent laboratory testing for secondary osteoporosis was negative, as were searches for causes of hypophosphatasemia other than HPP[14], including celiac disease, hypothyroidism, Cushing’s syndrome, multiple myeloma, anemia, or Zn++ or Cu++ deficiency[1,2]. Then (May 2016), genetic testing for HPP (University of Athens) revealed a heterozygous ALPL missense mutation (c.455 G>A→R135H). Notably, his serum PLP level measured with high-performance liquid chromatography was unexpectedly not elevated[13] but normal (19 µg/l; reference range 15-30); however, he was not investigated for hypovitaminosis B6[15].
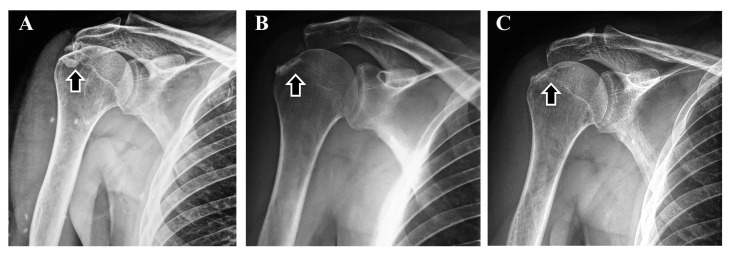
Figure 1.
A) Dec 2015: Before surgical removal, ectopic calcifications (arrow) in keeping with calcific periarthritis from hydroxyapatite crystal deposition are characteristic of adult HPP. B) Feb 2016: Two months after surgery, ectopic calcification has been partially removed. C) Feb 2021: Five years after surgery and 3.5 years of TPTD treatment, ectopic calcification is not increased.
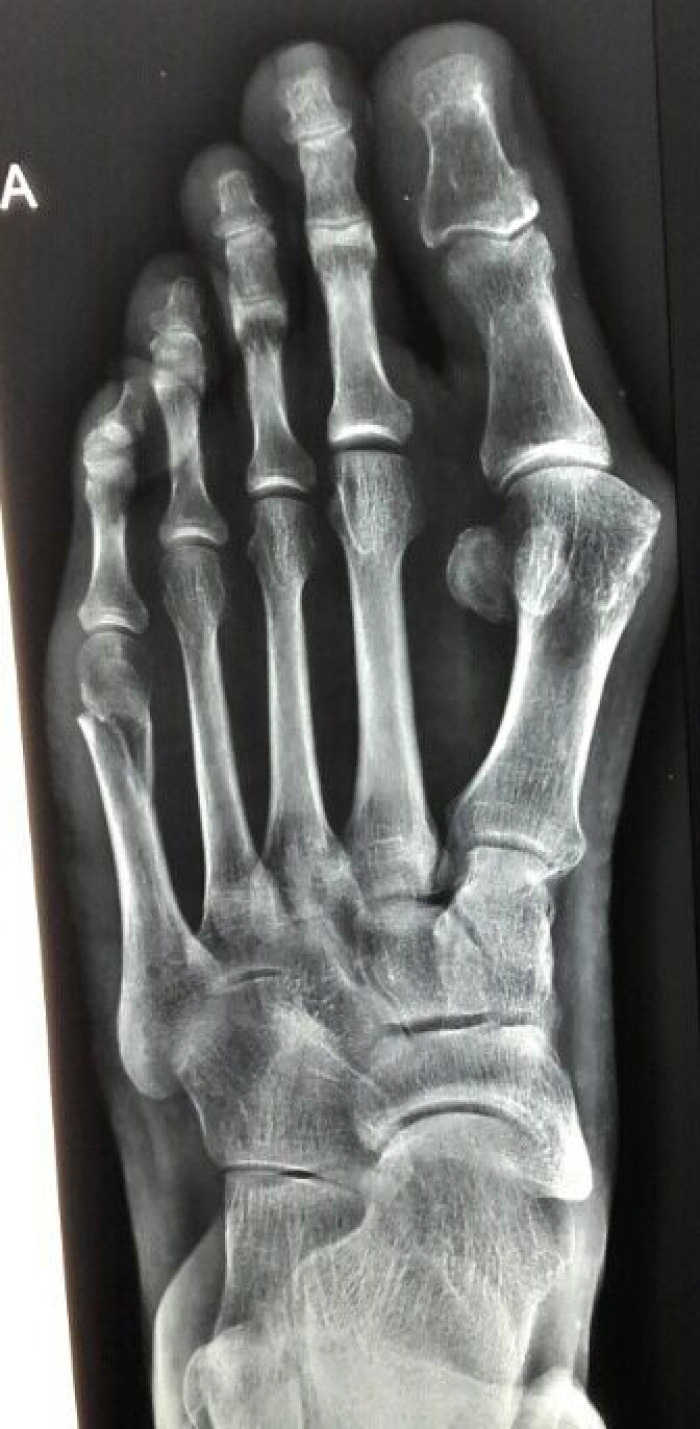
After our unsuccessful effort to have him participate in a clinical trial of AA for adult HPP, mainly because recruitment was completed at that time, we requested “off-label” use of TPTD from the Hellenic Organization for Medicines (EOF) and the patient’s medical insurance. Meanwhile, in March 2017, he experienced a new low-energy fracture of his left fifth metatarsal (Figure 2), with delayed healing of approximately 6 months.
Figure 2.
Mar 2017: This new low-energy fifth metatarsal fracture showed delaying healing, before TPTD initiation.
Then, with written consent by the patient as approved by the Ethics Committee of the School of Medicine, Aristotle University of Thessaloniki, Greece, TPTD treatment began (initially a daily subcutaneous dose of 20 µg/d) in June 2017. He received a stable dose of vitamin D3 (1200 IU/d) from the beginning of TPTD treatment. No calcium supplementation was provided, as his dietary intake was sufficient. Subsequently, a low-energy fracture at the left elbow occurred in August 2017. Both the new metatarsal and elbow fractures were managed conservatively.
Due to the limited number (n=730) of TPTD injections conventionally approved for osteoporosis treatment (2 years, when administered daily), from November 2017 TPTD injections (20 µg) were instead administered every other day (QOD).
After initiation of TPTD treatment, serum ALP activity did not increase in the long-term (33-48 U/l); calcium, phosphate, and PTH were essentially stable; and 25(OH)D remained sufficient. Bone ALP, measured with an enzyme-linked immunosorbent assay (MicroVue Quidel Corporation, San Diego, CA), increased slightly (40-50% of total ALP, whereas 35% pre-treatment). Serum PLP level was again normal (16 µg/l). After August 2017, no further fractures occurred and his height did not change. There was no tooth loss. He did not report improvement in his musculoskeletal pain, which remained mild.
Then, from his apparently diminished fracturing and no treatment alternatives, in March 2020, TPTD injections (20 µg) were administered every third day. In December 2020, his lumbar spine BMD was 1.141 g/cm2 (increased by 15%; Z-score -1.5) and femoral neck BMD was 0.766 g/cm2 (increased by 6%; Z-score -2.4). Radiographs of his right shoulder revealed no worsening of what calcinosis remained after his surgery (Figure 1C).
Since December 2020, TPTD injections (20 µg) have been administered once weekly to prolong the therapy. Key elements of his treatment history and response are summarized in Table 1.
Table 1.
Key elements of the patient’s treatment history and responses.
| Date (MM/YYYY) | Event | Height (cm) | ΒΜΙ (kg/m2) | ALP (IU/l) | Calcium (mg/dl) | Phosphate (mg/dl) | PTH (pg/ml) | 25(OH)D (ng/ml) | PLP (µg/l) | LS BMD (g/cm2) | FN BMD (g/cm2) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference range | 40-120 | 8.5-10.5 | 2.5-4.5 | 7-53 | >30 | 15-30 | |||||
| --/2011 | 45 | 10.2 | |||||||||
| --/2013 | 33 | 10.3 | |||||||||
| 11/2014 | 40 | 10.1 | 4.1 | ||||||||
| 03/2015 | 39 | 10.4 | |||||||||
| 09/2015 | Start alendronate treatment | 0.992 | 0.726 | ||||||||
| 12/2015 | Surgical removal of calcinosis (right shoulder) | 45 | 9.5 | 3.2 | |||||||
| 02/2016 | Presentation to us; alendronate discontinuation | 187 | 30.3 | 33 | 10.2 | 3.7 | 23.0 | 33.7 | |||
| 05/2016 | Genetic testing for HPP | 26.0 | 44.8 | 19 | |||||||
| 11/2016 | 37 | 10.4 | |||||||||
| 03/2017 | Low-energy fracture (left 5th metatarsal) | ||||||||||
| 06/2017 | Start TPTD treatment (once daily) | 33 | 10.0 | 3.5 | 25.0 | ||||||
| 08/2017 | Low-energy fracture (left elbow) | ||||||||||
| 11/2017 | Change TPTD treatment to every other day | 187 | 32.9 | 37 | 10.4 | 3.5 | |||||
| 12/2017 | 48 | 10 | |||||||||
| 02/2019 | 47 | 10.2 | 3.8 | 33.5 | 31.4 | ||||||
| 09/2019 | 187 | 29.2 | 39 | 9.8 | 3.0 | 16 | |||||
| 03/2020 | Change TPTD treatment to every third day | ||||||||||
| 12/2020 | Change TPTD treatment to once weekly | 187 | 29.5 | 42 | 10 | 3.4 | 32.0 | 32.5 | 1.141 | 0.766 | |
| 07/2021 | 42 | ||||||||||
Abbreviations: ALP, alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; FN, femoral neck; LS, lumbar spine; PLP, pyridoxal 5ʹ-phosphate; PTH, parathyroid hormone;
TPTD, teriparatide; 25(OH)D, 25-hydroxyvitamin D.
Discussion
Herein, we acted on evidence that our patient manifested adult HPP. He had suffered unexplained, recurrent, low-trauma, poorly-healing appendicular fractures with mild musculoskeletal pain suggestive of osteomalacia[2], and had calcific periarthritis[6]. Laboratory studies showed persisting hypophosphatasemia, low BMD, and in ALPL a heterozygous missense mutation associated with HPP (see below)[16]. Uniquely, he received TPTD treatment longer than two years, yet the total dosing (n=730) did not exceed the 2-year daily administration of TPTD approved for osteoporosis[9]. Now, post-marketing surveillance has shown that TPTD does not increase the 15-year risk of osteosarcoma[17]. Apart from one new low-energy fracture in the left elbow two months after TPTD initiation (i.e., early for TPTD to exert its anti-fracture efficacy), he had no further fractures and BMD improved predominantly in his lumbar spine. Serum ALP activity did not increase[13] or PLP level decrease, despite his having one intact ALPL allele[2]. Accordingly, perhaps TPTD administration led to small but therapeutic increases in TNSALP within his skeleton, yet not reflected in his serum ALP activity.
Administration of TPTD “off-label” for HPP has been reported[10,12,13], and was reviewed in 2016 by Camacho et al[11]. The first such patient was described in 2007, a 56-year-old woman with adult HPP who benefitted from a 18-month course of daily 20 µg TPTD injections[10]. Since then, approximately 15 patients with adult HPP have received TPTD, most for a maximum of 1.5 or 2 years, in keeping with its approval for postmenopausal osteoporosis or osteoporosis in men[9]. Our findings are novel in that TPTD administered every other day or every third day seemed to increase BMD and prevent new fractures, despite no apparent increase in serum total ALP activity. In fact, TPTD has been used in Japan once weekly for osteoporosis, albeit at a higher dose (56.5 µg/d), than daily administration (28.2 µg), and provided comparable efficacy[18]. Other authors reported that the same cumulative dose of TPTD given cyclically over 4 years has a similar effect on BMD compared with standard daily TPTD over 2 years[19]. It has been recently recommended that TPTD may be used for more than two years for patients with persisting or recurring high risk of fracture (https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=aae667c5-381f-4f92-93df-2ed6158d07b0&type=display). Thus, this alternative seems important for adults with HPP not treated with AA, since antiresorptives (e.g. bisphosphonates, denosumab) do not appear to be alternatives for TPTD, because further suppression of bone turnover and ALP activity is possible, unmasking or exacerbating underlying osteomalacia and possibly leading to atypical femoral fractures[14].
Our patient’s c.455 G>A→R135H missense change in ALPL is reportedly a benign polymorphism in the HPP mutation database (http://www.sesep.uvsq.fr/03_hypo_mutations.php), yet it is likely pathogenic, because it has been identified in severe autosomal recessive HPP. In fact, it was heterozygous in another of our adult HPP patients, as we reported in 2006[16]. Additionally, it was heterozygous in the mother of a child with lethal, likely autosomal recessive, HPP[20], although the child was not tested and the skeletal health of the mother was not known. The father was heterozygous for the ALPL mutation 535G>A→A162T, which, when homozygous, is associated with lethal HPP[20].
An important issue for managing our patient will emerge when he completes the 730 TPTD injections. Perhaps, we will request from the Hellenic Organization for Medicines (EOF) their approval for long-term off-label TPTD use, until AA is licensed for adult HPP in Europe, or until he can enter a new research protocol to treat HPP. Alternatively, we may ask permission for off-label treatment using the anti-sclerostin antibody romosozumab, which currently cannot be prescribed for longer than one year.
Some of our findings and conclusions have limitations. The patient’s circulating vitamin B6 (PLP) level was not elevated[21,22]; however, serum 4-pyridoxic acid was not assayed to assess if this reflected vitamin B6 deficiency[21,22]. PLP levels are lower in patients with mono-allelic ALPL defects, as in our patient, compared to bi-allelic mutations[22], but can be normal despite bi-allelic life-threatening HPP if there is dietary vitamin B6 insufficiency[15]. Our patient did not undergo bone scintigraphy to look for pseudofractures or have non-decalcified iliac crest histology after oral tetracycline “labeling” to document the defective skeletal mineralization and osteomalacia[23] expected in adult HPP[6]. Markers of bone turnover (e.g., osteocalcin, procollagen 1 N-terminal propeptide, C-terminal telopeptide) were not studied, and bone quality using peripheral quantitative computed tomography was not evaluated.
Thus, we treated a man with emerging adult HPP unconventionally; i.e., for 4 years using less frequent doses of TPTD than typically given for osteoporosis. Despite no improvement in serum total ALP activity or decrease in PLP, his BMD increased and no new fractures occurred after 2 months of this therapy. Our findings represent success in overcoming two prescription deadlocks; AA was denied for his adult HPP, and TPTD was not to be administered daily for more than two years. We suggest that for adult HPP, TPTD administration can be prolonged with clinical benefit.
Authors’ contributions
SAP: conception and design of the work, acquisition and interpretation of data; drafted the manuscript and revised it critically for important intellectual content; approved the version to be published. ST: interpretation of data; revised the manuscript critically for important intellectual content; approved the version to be published. AG: interpretation of data; revised the manuscript critically for important intellectual content; approved the version to be published. PK: acquisition of data; revised the manuscript critically for important intellectual content; approved the version to be published. MPW: conception and design of the work, interpretation of data; drafted the manuscript and revised it critically for important intellectual content; approved the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. SAP accept responsibility for the integrity of the data analysis and that author must be identified as such.
Funding
This work was funded in part by Shriners Hospitals for Children (#71004) and the Clark and Mildred Cox Inherited Metabolic Bone Disease Research Fund (#3574) at the Barnes-Jewish Hospital Foundation; St. Louis, MO, USA.
Footnotes
Symeon Tournis has received lecture and/or advisory board fees from Amgen, Eli Lilly, Vianex, ITF Hellas, Libytec, Merck Biopharma Greece, Galenica, and Shire. Michael P. Whyte consults for Aruvant Sciences, Inc., New York, NY. The remaining authors have nothing to declare.
Edited by: P. Makras
References
- 1.Millán JL, Whyte MP. Alkaline Phosphatase and Hypophosphatasia. Calcif Tissue Int. 2016;98(4):398–416. doi: 10.1007/s00223-015-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whyte MP. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12(4):233–46. doi: 10.1038/nrendo.2016.14. [DOI] [PubMed] [Google Scholar]
- 3.Whyte MP. Hypophosphatasia and how alkaline phosphatase promotes mineralization. In: Thakker RV, Whyte MP, Eisman J, Igarashi T, editors. In:Genetics of Bone Biology and Skeletal Disease. 2nd ed. San Diego, CA: Elsevier; 2018. pp. 481–504. [Google Scholar]
- 4.Taillandier A, Domingues C, Dufour A, Debiais F, Guggenbuhl P, Roux C, Cormier C, Cortet B, Porquet-Bordes V, Coury F, Geneviève D, Chiesa J, Colin T, Fletcher E, Guichet A, Javier RM, Laroche M, Laurent M, Lausch E, LeHeup B, Lukas C, Schwabe G, van der Burgt I, Muti C, Simon-Bouy B, Mornet E. Genetic analysis of adults heterozygous for ALPL mutations. J Bone Miner Metab. 2018;36(6):723–33. doi: 10.1007/s00774-017-0888-6. [DOI] [PubMed] [Google Scholar]
- 5.Genest F, Claußen L, Rak D, Seefried L. Bone mineral density and fracture risk in adult patients with hypophosphatasia. Osteoporos Int. 2021;32(2):377–85. doi: 10.1007/s00198-020-05612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guañabens N, Mumm S, Möller I, González-Roca E, Peris P, Demertzis JL, Whyte MP. Calcific periarthritis as the only clinical manifestation of hypophosphatasia in middle-aged sisters. J Bone Miner Res. 2014;29(4):929–34. doi: 10.1002/jbmr.2110. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein RS, Whyte MP. Fifty-year follow-up of hypophosphatasia. Arch Intern Med. 1981;141(12):1720–1. doi: 10.1001/archinte.141.12.1720. [DOI] [PubMed] [Google Scholar]
- 8.Whyte MP, Simmons JH, Moseley S, Fujita KP, Bishop N, Salman NJ, Taylor J, Phillips D, McGinn M, McAlister WH. Asfotase alfa for infants and young children with hypophosphatasia:7 year outcomes of a single-arm, open-label, phase 2 extension trial. Lancet Diabetes Endocrinol. 2019;7(2):93–105. doi: 10.1016/S2213-8587(18)30307-3. [DOI] [PubMed] [Google Scholar]
- 9.Anastasilakis AD, Polyzos SA, Yavropoulou MP, Makras P. Combination and sequential treatment in women with postmenopausal osteoporosis. Expert Opin Pharmacother. 2020;21(4):477–90. doi: 10.1080/14656566.2020.1717468. [DOI] [PubMed] [Google Scholar]
- 10.Whyte MP, Mumm S, Deal C. Adult hypophosphatasia treated with teriparatide. J Clin Endocrinol Metab. 2007;92(4):1203–8. doi: 10.1210/jc.2006-1902. [DOI] [PubMed] [Google Scholar]
- 11.Camacho PM, Mazhari AM, Wilczynski C, Kadanoff R, Mumm S, Whyte MP. Adult Hypophosphatasia Treated with Teriparatide:Report of 2 Patients and Review of the Literature. Endocr Pract. 2016;22(8):941–50. doi: 10.4158/EP15890.OR. [DOI] [PubMed] [Google Scholar]
- 12.Schalin-Jantti C, Mornet E, Lamminen A, Valimaki MJ. Parathyroid hormone treatment improves pain and fracture healing in adult hypophosphatasia. J Clin Endocrinol Metab. 2010;95(12):5174–9. doi: 10.1210/jc.2010-1168. [DOI] [PubMed] [Google Scholar]
- 13.Gagnon C, Sims NA, Mumm S, McAuley SA, Jung C, Poulton IJ, Ng KW, Ebeling PR. Lack of sustained response to teriparatide in a patient with adult hypophosphatasia. J Clin Endocrinol Metab. 2010;95(3):1007–12. doi: 10.1210/jc.2009-1965. [DOI] [PubMed] [Google Scholar]
- 14.Sutton RA, Mumm S, Coburn SP, Ericson KL, Whyte MP. “Atypical femoral fractures”during bisphosphonate exposure in adult hypophosphatasia. J Bone Miner Res. 2012;27(5):987–94. doi: 10.1002/jbmr.1565. [DOI] [PubMed] [Google Scholar]
- 15.Whyte MP, May JD, McAlister WH, Burgener K, Cortez SR, Kreienkamp R, Castro O, Verzola R, Zavala AS, McPherson CC, Gottesman GS, Ericson KL, Coburn SP, Arbelaez AM. Vitamin B(6) deficiency with normal plasma levels of pyridoxal 5'-phosphate in perinatal hypophosphatasia. Bone. 2021;150:116007. doi: 10.1016/j.bone.2021.116007. [DOI] [PubMed] [Google Scholar]
- 16.Khandwala HM, Mumm S, Whyte MP. Low serum alkaline phosphatase activity and pathologic fracture:case report and brief review of hypophosphatasia diagnosed in adulthood. Endocr Pract. 2006;12(6):676–81. doi: 10.4158/EP.12.6.676. [DOI] [PubMed] [Google Scholar]
- 17.Gilsenan A, Midkiff K, Harris D, Kellier-Steele N, McSorley D, Andrews EB. Teriparatide Did Not Increase Adult Osteosarcoma Incidence in a 15-Year US Postmarketing Surveillance Study. J Bone Miner Res. 2021;36(2):244–51. doi: 10.1002/jbmr.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugimoto T, Shiraki M, Fukunaga M, Kishimoto H, Hagino H, Sone T, Nakano T, Ito M, Yoshikawa H, Minamida T, Tsuruya Y, Nakamura T. Study of twice-weekly injections of Teriparatide by comparing efficacy with once-weekly injections in osteoporosis patients:the TWICE study. Osteoporos Int. 2019;30(11):2321–31. doi: 10.1007/s00198-019-05111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cosman F, Nieves JW, Roimisher C, Neubort S, McMahon DJ, Dempster DW, Lindsay R. Administration of teriparatide for four years cyclically compared to two years daily in treatment Naïve and alendronate treated women. Bone. 2019;120:246–53. doi: 10.1016/j.bone.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Taillandier A, Lia-Baldini AS, Mouchard M, Robin B, Muller F, Simon-Bouy B, Serre JL, Bera-Louville A, Bonduelle M, Eckhardt J, Gaillard D, Myhre AG, Kortge-Jung S, Larget-Piet L, Malou E, Sillence D, Temple IK, Viot G, Mornet E. Twelve novel mutations in the tissue-nonspecific alkaline phosphatase gene (ALPL) in patients with various forms of hypophosphatasia. Hum Mutat. 2001;18(1):83–4. doi: 10.1002/humu.1154. [DOI] [PubMed] [Google Scholar]
- 21.Schini M, Nicklin P, Eastell R. Establishing race-, gender- and age-specific reference intervals for pyridoxal 5'-phosphate in the NHANES population to better identify adult hypophosphatasia. Bone. 2020;141:115577. doi: 10.1016/j.bone.2020.115577. [DOI] [PubMed] [Google Scholar]
- 22.Whyte MP, Coburn SP, Ryan LM, Ericson KL, Zhang F. Hypophosphatasia:Biochemical hallmarks validate the expanded pediatric clinical nosology. Bone. 2018;110:96–106. doi: 10.1016/j.bone.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 23.Coe JD, Murphy WA, Whyte MP. Management of femoral fractures and pseudofractures in adult hypophosphatasia. J Bone Joint Surg Am. 1986;68(7):981–90. [PubMed] [Google Scholar]