Abstract
Monoclonal antibodies (mAbs) are gaining significant momentum as novel therapeutics for infections caused by antibiotic-resistant bacteria. We evaluated the mechanism by which antibacterial mAb therapy protects against Acinetobacter baumannii infections. Anticapsular mAb enhanced macrophage opsonophagocytosis and rescued mice from lethal infections by harnessing complement, macrophages, and neutrophils; however, the degree of bacterial burden did not correlate with survival. Furthermore, mAb therapy reduced proinflammatory (interleukin-1β [IL-1β], IL-6, tumor necrosis factor-α [TNF-α]) and anti-inflammatory (IL-10) cytokines, which correlated inversely with survival. Although disrupting IL-10 abrogated the survival advantage conferred by the mAb, IL-10–knockout mice treated with mAb could still survive if TNF-α production was suppressed directly (via anti–TNF-α neutralizing antibody) or indirectly (via macrophage depletion). Thus, even for a mAb that enhances microbial clearance via opsonophagocytosis, clinical efficacy required modulation of pro- and anti-inflammatory cytokines. These findings may inform future mAb development targeting bacteria that trigger the sepsis cascade.
Keywords: monoclonal antibody, gram-negative bacterial infection, Acinetobacter, cytokines, passive immunity, innate immunity
It has long been presumed that passive immunization clears bacterial infections by reducing bacterial burden through opsonophagocytosis. Here, we show that phagocyte cytokine modulation is the key mechanism by which an antibacterial monoclonal antibody resolves infections caused by Acinetobacter baumannii.
As drug-resistant pathogens continue to spread across the globe, the antibiotics pipeline intended to combat this threat is becoming more tenuous and less relevant [1, 2]. Experts have called for new approaches to combat antibiotic-resistant pathogens [3–7]. We recently described an anticapsular mAb targeting extremely drug-resistant Acinetobacter baumannii, which protected mice from lethal infection, even at submicrogram doses [8]. mAb-based treatments are a promising way to improve outcomes without driving resistance to antibiotics.
Historically, clinical efficacy of antibacterial mAbs and other passive immunization therapies has largely been presumed to depend on enhancing microbial clearance from the host by driving opsonophagocytosis and activating complement [9]. Indeed, our murine mAb enhanced opsonophagocytosis ex vivo by a human macrophage cell line and complement-mediated killing [8]. However, experts have recently theorized that other mechanisms of mAb efficacy may be just as important [9]. mAbs that effectively enhance clearance of bacteria have generally not been investigated previously for the potential impact on inflammatory modulation, recognizing only microbial clearance as their mechanism of action. However, some investigators have shown that mAbs can drive efficacy by altering the inflammatory milieu in the host, even if they do not affect microbial clearance [10–13]. We sought to determine what drives the efficacy of an anti-A. baumannii mAb, whether by enhancing microbial clearance or other factors mediating efficacy.
METHODS
Strains
HUMC1 is an A. baumannii clinical blood and lung isolate (from a patient with bacteremic ventilator-associated pneumonia), resistant to all antibiotics except colistin, and highly virulent in animal models [14–17].
C3HeB/Fe (wild type; strain No. 000658) mice were purchased from Jackson Laboratories and depleted of innate effectors as described below for survival, bacterial burden, and cytokine studies. Mice were between 9 and 12 weeks of age at the time of infection and weighed approximately 30 g.
C57BL/6 (wild type; strain No. 000664) mice were purchased from Jackson Laboratories. Mice were between 8 and 11 weeks of age at the time of infection. Congenic complement C3 knockout (C3-KO; strain No. 029661), interleukin-10 knockout (IL-10–KO; strain No. 002251), and tumor necrosis factor-α knockout (TNF-α–KO; strain No. 005540) mice were purchased from Jackson Laboratories. Mice were between 8 and 11 weeks of age at the time of infection and weighed approximately 30 g.
Bacterial Inoculum Preparation
Bacteria were grown overnight and passaged to log growth in tryptic soy broth before rinsing in phosphate-buffered saline (PBS) prior to infection. To reduce variability between inocula, identical aliquots of PBS-suspended subcultures were stored frozen at −80°C, thawed for use when needed, and diluted with PBS to the appropriate concentration, as we have previously described [18]. During all in vivo experiments, the treatment was administered within 1 hour after infection.
In Vitro Complement Susceptibility Assay
To evaluate complement susceptibility, A. baumannii was inoculated into 12-well tissue culture plate with Hank’s buffered salt solution (HBSS) with or without human serum (Innovative Research), either complement active or heat inactivated at 56°C for 30 minutes. Twenty micrograms of isotype control (placebo) or humanized C8 mAb were added. The plate was then incubated for 1 hour at 37°C and bacterial density was subsequently quantified by serially diluting cultures.
Ex Vivo Macrophage Opsonophagocytosis Assay
To evaluate opsonophagocytosis, we used ex vivo macrophages differentiated from human peripheral blood mononuclear cells (PBMC). We have shown that this quantifies phagocytosis/uptake of bacteria as opposed to surface binding [19]. Fresh human whole blood was combined with sodium heparin in a Vacutainer Mononuclear Cell Preparation Tube (Becton Dickinson). PBMC were isolated according to the manufacturer’s protocol, adhered to glass cover slips, and rinsed with PBS supplemented with 2% heat-inactivated fetal bovine serum. PBMC were then differentiated to macrophages with fresh 1.5 mL/well ImmunoCult-SF Macrophage Medium (STEMCELL) supplemented with 50 μg/mL recombinant human macrophage colony-stimulating factor and 50 ng/mL recombinant human interferon-γ (STEMCELL). A. baumannii was added to the wells in the presence of HBSS and serum (either complement active or not), with isotype control or C8 mAb. For staining, macrophages were washed 3 times with HBSS, fixed with 100% methanol, and Hema-3 stained according to the manufacturer’s protocol (Thermo Fisher Scientific).
Infection Model
Bacteria were administered intravenously (IV) via the tail vein, as we have previously described for A. baumannii [14–16, 20]. We have previously reported that A. baumannii does not extravasate from the endovascular compartment to invade tissues during bacteremia, contrary to numerous other pathogens we have studied [21, 22]. Thus, analysis of bacterial burden focused on the blood.
The mAb used to treat mice was previously found to mediate substantial protection against A. baumannii infection in mice [8]. We previously published on bacterial burden, cytokine output, and survival of nondepleted, intact mice infected with A. baumannii HUMC1 and treated with PBS [17, 21, 23] or an isotype control antibody [8], finding that none of these parameters were affected by the isotype control antibody. In addition to this extensive prior work, we also include a repeat validation experiment with an IgG isotype control (Thermo Fisher Scientific, catalog No. MAB002), allowing for substitution of PBS in vivo as the placebo.
In Vivo Depletion Studies
For complement depletion, we injected 100 µL of cobra venom factor (CVF; Complement Technology) intraperitoneally (IP) at 150 μg/mL to achieve a dose of 0.5 μg CVF per gram 2 days prior to infection [17]. To deplete macrophages, we injected IP 300 µL of 5 mg/mL liposomal clodronate (LC; Foundation Clodronate Liposomes) to achieve 50 μg LC per gram mouse mass (eg, a 30-g mouse received 300 μL or 1.5 mg LC) 2 days prior to infection. We confirmed macrophage depletion in vivo by flow cytometry [17].
Finally, for neutrophil depletion, we administered cyclophosphamide (Baxter) IP at 230 mg/kg. With use of cyclophosphamide, neutrophils are fully depleted 24–48 hours postinjection and return to normal levels 1 week postinjection [17]. While cyclophosphamide also causes minor short-term depletion of monocytes from the blood, it does not deplete long-lived, tissue-resident macrophages [24]. Cyclophosphamide was chosen for neutrophil depletion because it has been previously shown that the alternative (antibody-mediated neutrophil depletion) relies on functional macrophages to be present [25]. Thus, the use of antibodies to deplete neutrophils would have precluded double- and triple-depletion studies, which were essential to defining the mAb’s mechanism of protection.
C3a and C5a Enzyme-Linked Immunosorbent Assay
To detect the soluble active complement components C3a and C5a in the serum of infected mice, we performed an enzyme-linked immunosorbent assay (ELISA), as described [26, 27]. Standards were set up by adding 100 μL/well recombinant mouse complement component C3a (R&D; catalog No. 8085-C3-025) or C5a (R&D, catalog No. 2150-C5) protein at 128 μg/mL and serially diluting 2-fold to 2 μg/mL for a range of values.
F(ab′)2Fragments
F(ab′)2 fragments were generated with the enzyme ficin using a kit (Thermo Fisher Scientific; catalog No. 44980) and confirmed to bind to the capsular polysaccharide surface of A. baumannii by flow cytometry.
Statistical Analysis
Given the nonparametric nature of the data, bacterial burden and cytokines are presented as medians with interquartile ranges. Survival was compared by the nonparametric log-rank test with α = .05. Cytokine concentrations and survival were compared by the Spearman rank-order correlation coefficient (ρ) with α = .05.
Study Approval
All animal experiments were approved by the Institutional Committee on the Use and Care of Animals (IACUC) at the Keck School of Medicine at the University of Southern California, following the National Institutes of Health guidelines for animal housing and care (IACUC protocols 20208 and 20750).
RESULTS
Humanized mAb Mediates Opsonophagocytosis and Complement Activity
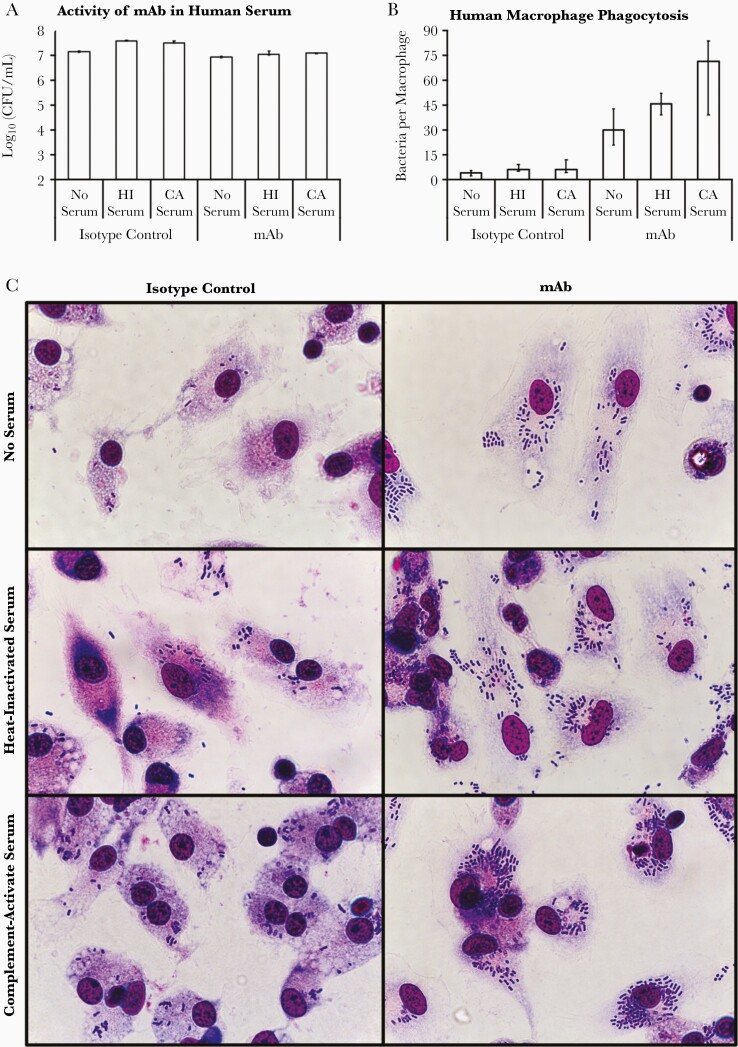
To evaluate the relevance of our lead mAb to human immunotherapy, we first assessed how it impacted the activity of human innate effectors (complement and macrophages) against bacteria. Human serum did not inhibit the growth of the A. baumannii strain HUMC1, a carbapenem-resistant, extremely drug-resistant clinical isolate known to be resistant to complement [14–17], regardless of whether complement was active or heat inactivated (Figure 1A). Addition of mAb restricted bacterial expansion but did not reduce bacterial density, with or without complement-active serum (Figure 1A).
Figure 1.
mAb mediates opsonophagocytosis and activates complement. A, The complement-resistant Acinetobacter baumannii strain HUMC1 was best able to grow in the presence of human serum (complement active or heat inactivated) but was hindered from growing with the addition of mAb to serum. B, Human macrophages were considerably better at taking up bacteria in the presence of mAb, in a manner enhanced by complement. C, Micrographs of human macrophages with internalized A. baumannii representative of the group’s median. The magnification is 1000x oil-immersion. A and B, Plots show medians and interquartile ranges. Abbreviations: CA, complement active; CFU, colony-forming unit; HI, heat inactivated; mAb, monoclonal antibody.
However, the mAb opsonized bacteria for significantly enhanced phagocytosis by human macrophages derived from freshly obtained PBMC, in a manner synergistically enhanced by human complement-active serum (Figure 1B and 1C).
Contribution of Innate Effectors to Survival in the Setting of mAb Therapy
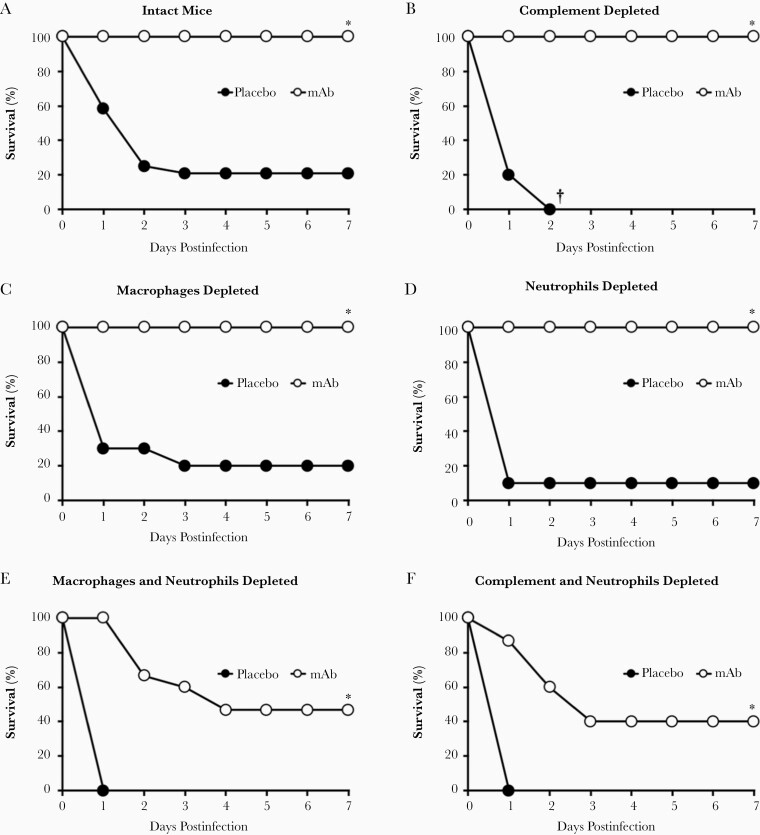
We next selectively depleting wild-type C3HeB/Fe mice of their innate immune effectors: complement, macrophages, and/or neutrophils. We then infected the mice IV with A. baumannii and treated them with the anti-A. baumannii mAb or placebo. The vast majority of mice died without mAb therapy, regardless of whether their immune system was intact or depleted of one or more innate effectors (Supplementary Figure 1). In the absence of mAb therapy, complement-depleted mice died significantly faster than macrophage- or neutrophil-depleted mice (black circles in Figure 2A–2D). However, mAb therapy rescued all singly depleted mice from death (white circles in Figure 2A–2D).
Figure 2.
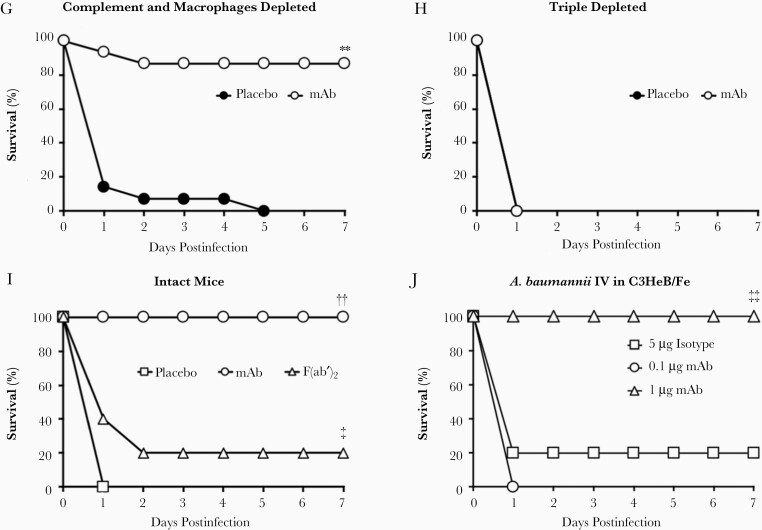
Depleting innate effectors altered mAb efficacy in mice infected with Acinetobacter baumannii. Mice (n = 10–24 per group) were depleted of 0, 1, 2, or 3 innate effectors, infected IV with 2×107 CFU A. baumannii HUMC1, and treated with placebo or 5 μg mAb. Without mAb therapy (placebo-treated mice), single depletion of macrophages (C) or neutrophils (D) resulted in survival similar to intact mice (A). However, single depletion of complement (B), double depletions (E–G), and triple depletion (H) led to 100% fatality after A. baumannii infection in the absence of mAb therapy. mAb therapy resulted in complete protection of intact (A) and singly depleted (B–D) mice, as well as substantial protection of mice doubly depleted of complement and macrophages (G). mAb-treated mice double-depleted of neutrophils and macrophages (E) or neutrophils and complement (F) had significantly better survival than mAb-treated mice depleted of all 3 innate effectors (H). I, Mice (n = 5 per group) were infected IV with 2×107 CFU A. baumannii HUMC1 and treated with placebo, 1 μg mAb, or 1 μg F(ab′)2. J, Mice (n = 5 per group) were infected IV with 2×107 CFU A. baumannii HUMC1, and treated with 5 μg isotype control antibody, 1 μg mAb, or 0.1 μg mAb. Data presented in Figure 2, Figure 3, and Figure 5 were collected from the same set of mice; *P < .05 compared to placebo-treated intact, macrophage-depleted, and neutrophil-depleted mice; **P < .05 compared to mAb-treated mice depleted of neutrophils and complement, neutrophils and macrophages, or all 3 innate effectors; †P < .05 compared to mAb-treated triple-depleted mice; ††P < .05 compared to placebo- and F(ab′)2-treated mice; ‡P < .05 compared to placebo-treated mice; ‡‡P < .05 compared to isotype control-treated mice. Abbreviations: CFU, colony-forming unit; IV, intravenously; mAb, monoclonal antibody.
All doubly depleted control mice succumbed to infection. In contrast, those treated with mAb significantly improved survival across all 3 groups (Figure 2E–2G). Notably, doubly depleted mice treated with mAb survived much better when only neutrophils remained (87% survival) compared to mice with only complement (47% survival) or macrophages (40% survival) remaining (Figure 2G versus Figure 2E and 2F). Mice depleted of all 3 effectors died regardless of whether they received mAb therapy, highlighting mAb reliance on innate effectors for efficacy (Figure 2H). Enzymatic cleavage of the Fc region eliminated the mAb’s efficacy, as demonstrated by intact mice unable to survive A. baumannii infection when treated with the resultant F(ab′)2 fragments (Figure 2I). Finally, the lower limit of protection was found to be above 0.1 μg mAb/mouse with 1 μg mAb/mouse providing full protection versus mice treated with isotype control antibody (Figure 2J) and 0.5 μg mAb/mouse providing some protection (70% survival) [8].
These results underscore the functional importance and redundancy of innate effectors for surviving A. baumannii bacteremia, highlight the importance of the Fc region, and implicate neutrophils as the most potent innate effector in the presence of mAb.
Contribution of Innate Effectors to Bacterial Clearance in the Setting of mAb Therapy
Having established the impact of innate effectors on survival, we next sought to determine the contribution of each effector toward bacterial clearance at 2 hours (Figure 3A and 3B) and 9 hours (Figure 3C and 3D) postinfection in placebo- and mAb-treated mice. Bacterial burden in placebo-treated mice decreased with complement depletion, increased with macrophage depletion, and was unaltered by neutrophil depletion (Figure 3E).
Figure 3.
Mice depleted of innate effectors and treated with mAb C8 were able to maintain or reduce bacterial burden between 2 and 9 hours postinfection; however, some still succumbed to the infection. Mice were depleted of 0, 1, 2, or 3 innate effectors, infected IV with 2×107 CFU Acinetobacter baumannii HUMC1, and treated with placebo or 5 μg mAb (same set of mice as in Figure 2 and Figure 5). Mice were evaluated for bacterial burden in the blood at 2 hours postinfection (A) and 9 hours postinfection (C). Change in bacterial density with mAb treatment at 2 hours postinfection (B) and 9 hours postinfection (D). E, Change in bacterial burden over time, subtracting log-transformed bacterial densities in mice at 2 hours postinfection from levels in mice at 9 hours postinfection. n = 4–9 mice per group; multiple experiments combined. Gray shading indicates mouse groups that achieved >80% survival as shown in Figure 1. F, Mice were infected IV with 2×107 CFU A. baumannii HUMC1 and treated with placebo, 1 μg mAb, or 1 μg F(ab′)2. P < .05 compared to placebo- and F(ab′)2-treated mice. All plots show medians and interquartile ranges. Abbreviations: CFU, colony-forming unit; IV, intravenously; mAb, monoclonal antibody.
mAb therapy dramatically reduced bacterial burden across all groups compared to placebo treatment, including mice depleted of all 3 effectors (Figure 3B and 3D). However, mAb therapy only reduced bacterial burden if the Fc was present, as F(ab′)2 fragments did not mediate bacterial reduction (Figure 3F).
By 9 hours postinfection, bacterial burden in the setting of mAb therapy remained unaltered by neutrophil depletion, but increased with macrophage and complement depletion—similar to placebo-treated mice (Figure 3C). In contrast, both groups of mAb-treated, doubly depleted mice lacking neutrophils experienced 100-fold increases of bacterial densities in their blood compared to nondepleted intact mice by 9 hours postinfection (Figure 3C). But doubly depleted mice retaining neutrophils did not experience such increased bacterial burden (Figure 3C). Thus, despite neutrophils being the single most important innate effector to drive mAb clinical efficacy with survival as the end point (Figure 2E–2G), neutrophils appeared expendable for mAb-mediated reduction in bacterial density of singly depleted mice. These results indicate complex functional redundancy and suggest an alternative mechanism driving efficacy aside from simply opsonizing for enhanced phagocytosis.
The possibility of an alternative mechanism for mAb efficacy was further reinforced by the discordance between bacterial density and survival of infected mice. Specifically, despite the fact that all dual- and triple-depleted groups treated with mAb had 300- to 70 000-fold lower bacterial densities compared to placebo-treated controls by 9 hours postinfection, all but the doubly depleted group retaining neutrophils had very high mortality (Figure 2E–2H). Thus, neutrophils were key to mAb-mediated mouse survival, as mAb efficacy was lost in neutrophil-depleted mice even though the mAb still reduced bacterial density in the blood of those mice. Bacterial burden therefore did not correlate with survival, and mice succumbed to the infection despite the mAb effectively reducing bacterial burden.
Evaluating the change in bacterial density from 2 to 9 hours postinfection among placebo- and mAb-treated groups further uncouples bacterial burden from survival (Figure 3E). Despite static blood bacterial density over the 2 time points (Figure 3E), 80%–90% of placebo-treated intact and neutrophil-depleted mice died (Figure 2A and 2D). However, placebo-treated mice depleted of macrophages sustained >80-fold increases in bacterial burden over that period (Figure 3E) while experiencing the same mortality (Figure 2C). In fact, by 9 hours postinfection all placebo-treated mice depleted of macrophages had bacterial burdens 30- to 150-fold greater than intact mice (Figure 3C), highlighting the role macrophages play in restraining the growth of bacteria in the absence of mAb therapy, even though depleting macrophages did not result in worse survival than intact mice.
Collectively, these results indicate that bacterial density correlates poorly with survival, even though it correlates strongly with the presence of macrophages in placebo-treated mice and neutrophils in mAb-treated mice.
Factor C3 Is Required for Complement to Mediate Protection in the Setting of mAb Therapy
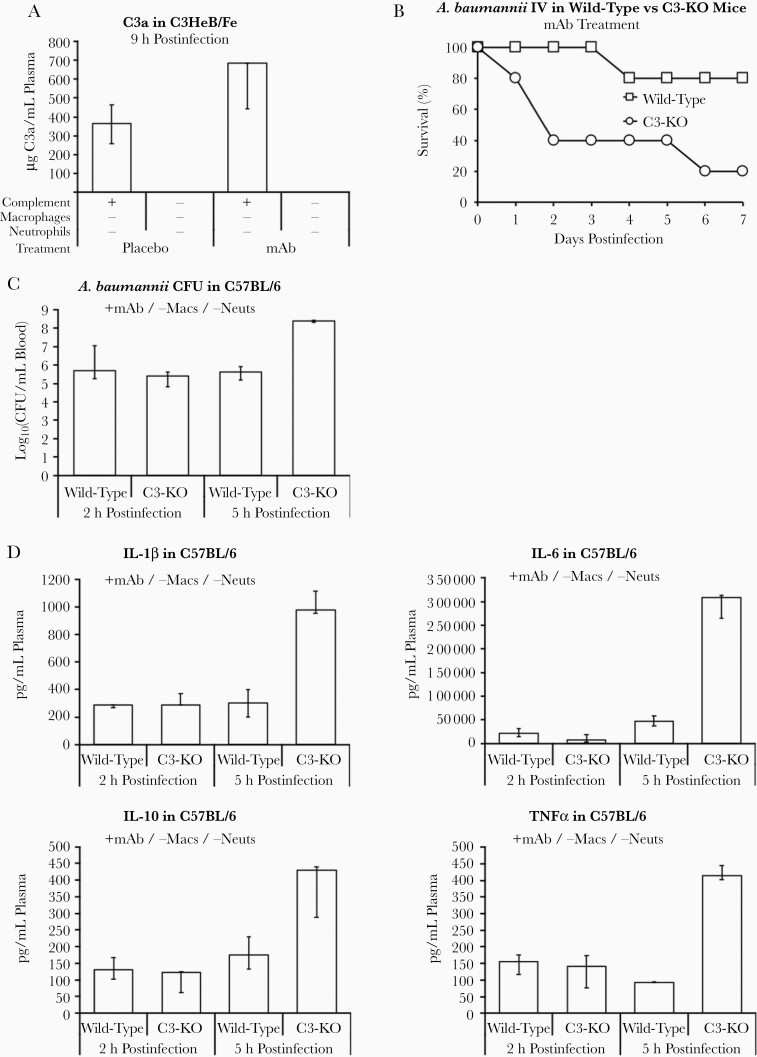
We found that C5a was undetectable in serum from mice of all groups, but C3a serum concentrations in mice treated with mAb were double those in the serum of placebo-treated mice (Figure 4A). Knocking out C3 abrogated mAb efficacy in mice, demonstrating that complement required component C3 for efficacy (Figure 4B). Bacterial burden in mAb-treated C3-KO mice was similar to wild-type mice at 2 hours postinfection (Figure 4C). However, by 5 hours postinfection the C3-KO bacterial burden increased by 2.7-log10 compared to wild-type mice (Figure 4C). The canonical proinflammatory (IL-1β, IL-6, and TNF-α) and anti-inflammatory (IL-10) cytokines were similarly elevated from baseline in wild-type and C3-KO mice at 2 hours postinfection. However, again by 5 hours postinfection C3-KO mice experienced cytokine storm with 2.5–6.6 times as much inflammatory cytokine output compared to wild-type mice (Figure 4D).
Figure 4.
mAb modulates inflammation through complement C3 to improve survival during Acinetobacter baumannii bacteremia. A, Mice (n = 5–8 mice per group) were depleted of macrophages and neutrophils, infected IV with 2×107 CFU A. baumannii HUMC1, and treated with placebo or 5 μg mAb. ELISA of complement C3a shows that mice were able to convert more C3 to C3a when treated with mAb compared to placebo (same mice as in Figure 2E and 2H). B–D, Wild-type and C3-KO mice (n = 5 per group) were depleted of macrophages and neutrophils, infected IV with 1×107 CFU A. baumannii HUMC1, and treated with 5 μg mAb. B, Knocking out C3 considerably diminished mAb-mediated protection from A. baumannii infection. Blood was harvested at 2 and 9 hours postinfection and plasma cytokine levels were determined by multiplex Luminex assay. C, Bacterial burden in mAb-treated mice was similar among the 2 groups at 2 hours postinfection but by 5 hours postinfection CFU/mL blood had increased only in the C3-KO mice. D, Likewise, pro- and anti-inflammatory cytokines were similar among the 2 groups at 2 hours postinfection but again by 5 hours postinfection cytokines had increased only in the C3-KO mice. A, C, and D, Plots show medians and interquartile ranges. Abbreviations: CFU, colony-forming unit; ELISA, enzyme-linked immunosorbent assay; IL, interleukin; IV, intravenously; KO, knock out; mAb, monoclonal antibody; Macs, macrophages; Neuts, neutrophils.
The Role of Modulating Inflammation in mAb Efficacy
The disconnect between survival (Figure 2) and bacterial density (Figure 3) across multiple groups of mice strongly suggested that mAb therapy improves survival by a mechanism other than exclusively reducing bacterial burden. We had previously found a similar result in diabetic mice where survival was driven more by changes to inflammatory cytokines than bacterial burden [23]. We therefore investigated the impact of mAb therapy on cytokine output.
In placebo-treated mice, IL-1β and IL-6 levels were unaltered by effector depletion at 2 hours postinfection (Figure 5). In contrast, groups that were depleted of macrophages experienced significantly elevated IL-10 levels without mAb therapy, whereas neutrophil-depleted mice had lower IL-10 levels (Figure 5). In the presence of mAb therapy, neutrophil-depleted mice also had reduced levels of IL-10 compared to other groups with neutrophils intact. mAb therapy markedly reduced IL-10 across all groups compared to placebo-treated mice, except for triple-depleted mice at 9 hours postinfection, which had higher levels than their placebo-treated counterparts (Figure 5). Furthermore, all groups with ≥40% survival had lower IL-10 levels by 9 hours postinfection compared to groups with <40% survival.
Figure 5.
mAb therapy ameliorates pro- and anti-inflammatory cytokine concentrations in blood during Acinetobacter baumannii bacteremia. Mice were depleted of 0, 1, 2, or 3 innate effectors, infected IV with 2×107 CFU A. baumannii HUMC1, and treated with placebo or 5 μg mAb (same set of mice as in Figure 2 and Figure 3). Blood was harvested at 2 (left) or 9 hours (right) and plasma cytokine levels were determined by multiplex Luminex assay. n = 4–9 mice per group; multiple experiments combined. Gray shading indicates mouse groups that achieved >80% survival, as shown in Figure 2. All plots show medians and interquartile ranges. Abbreviations: CFU, colony-forming unit; IL, interleukin; IV, intravenously; mAb, monoclonal antibody; TNF-α, tumor necrosis factor-α.
The results for TNF-α were opposite to those of IL-10 with respect to effector disruption at 2 hours postinfection. Specifically, TNF-α levels were lowest in mice depleted of macrophages and highest in neutrophil-depleted mice, with mAb therapy decreasing TNF-α only in the absence of macrophages (Figure 5). Thus, macrophages appeared to be the predominant source of TNF-α, while neutrophils were the predominant source of IL-10. Finally, similar to IL-10 at 9 hours postinfection, all groups of mice experiencing ≥40% survival had lower TNF-α levels than all groups of mice sustaining <40% survival. Thus, lower TNF-α levels also appeared to correlate with better survival.
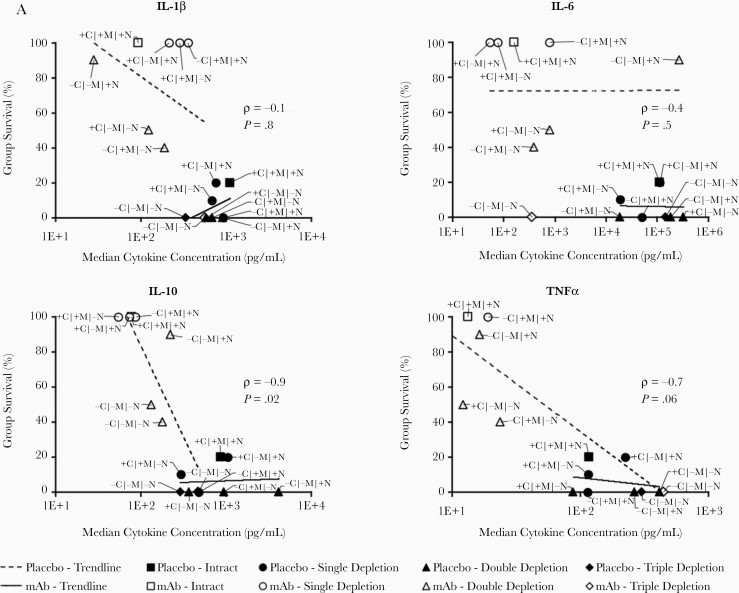
Furthermore, the antagonistic cytokines IL-10 and TNF-α were strongly inversely correlated with survival (Figure 6A), leading us to hypothesize that mAb-mediated protection was dependent on them. Mice in this model of bacteremia are known to die of sepsis [17, 20], so we hypothesized that mAb-mediated induction of proinflammatory TNF-α from macrophages leads to a hyperinflammatory state, which is neutralized by mAb-induced IL-10 from neutrophils.
Figure 6.
IL-10 but not TNF-α is required for mice to survive lethal bacteremia with mAb therapy. A, Median cytokine concentrations at 9 hours postinfection for each group of mice (n = 8 mice per group) graphed against the survival for that group. White-filled symbols indicate mAb-treated groups and black-filled symbols are placebo groups. Trend lines are separately shown for mAb-treated and placebo mice. Spearman rank correlation coefficient (ρ) and P value are shown for the correlations between cytokine concentrations and survival for mAb-treated mice. B, TNF-α–KO and wild-type mice survived IV infection with 1×108 CFU Acinetobacter baumannii HUMC1 when treated with 5 μg mAb, whereas IL-10–KO mice could not be rescued (C); *P < .05 compared to all other groups. Blood was harvested at 7 hours postinfection to quantify bacterial burden in the blood and obtain plasma cytokine levels by multiplex Luminex assay. D, Bacterial burden in mAb-treated mice was no different in IL-10–KO than in wild-type mice; however, only the mAb-treated wild-type group survived. E, Cytokine analysis revealed that IL-10–KO mice generated higher levels of proinflammatory cytokines than wild-type mice in the same treatment group (n = 5 mice per group). D and E, Plots show medians and interquartile ranges. Abbreviations: −, depleted; +, not depleted; C, complement; CFU, colony-forming unit; IL, interleukin; IV, intravenously; KO, knockout; M, macrophages; mAb, monoclonal antibody; N, neutrophils; TNF-α, tumor necrosis factor-α.
mAb Efficacy in Cytokine-Disrupted Mice
To test this hypothesis, we assessed survival in cytokine knockout mice. We first compared survival of wild-type C57BL/6 and congenic TNF-α–KO mice infected IV with A. baumannii and treated with placebo or mAb. As expected, there was no survival difference among genotypes. Knocking out proinflammatory TNF-α did not alter the survival of mice treated with mAb, demonstrating that TNF-α is not required for mAb efficacy (Figure 6B). TNF-α merely correlated with survival as sicker mice expressed more TNF-α, while healthier mice expressed less.
In contrast, disruption of IL-10 completely abrogated mAb-mediated efficacy (Figure 6C). Treatment with mAb completely protected wild-type mice as before but was ineffective at rescuing IL-10–KO mice, with mAb- and placebo-treated IL-10–KO mice succumbing to the infection simultaneously (Figure 6C). Thus, IL-10 is required for mAb-mediated efficacy during bacteremia.
To better understand why mAb therapy requires IL-10 for efficacy, we evaluated bacterial burden and cytokines at 7 hours postinfection. IL-10–KO mice had bacterial densities at or below the level of treatment-matched wild-type mice (Figure 6D), again underscoring the dissociation between bacterial burden and survival. Cytokines again clearly distinguished between the group that survived and the groups that succumbed to the infection (Figure 6E). Specifically, the proinflammatory cytokines IL-1β, IL-6, and TNF-α were elevated in placebo-treated mice and mAb-treated IL-10–KO mice, which did not survive (Figure 6E).
mAb Protects by Counteracting TNF-α from Macrophages
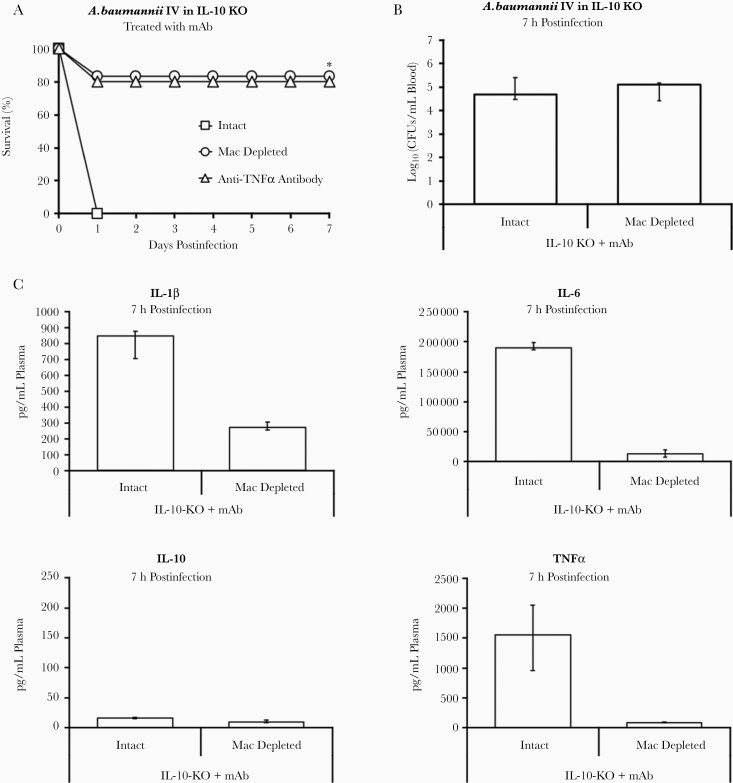
After demonstrating that IL-10 was required for mAb efficacy (Figure 6C), that high levels of TNF-α inversely correlated with survival (Figure 6A), and that the presence of macrophages was associated with worse mortality, we hypothesized that the mAb acted by inducing IL-10 from neutrophils to counteract TNF-α and rescue the host. To test this, we infected 3 groups of IL-10–KO mice and treated them with mAb: intact mice, a group depleted of macrophages, and another intact group treated with anti–TNF-α antibody. As before, IL-10–KO mice treated with mAb were unable to survive, but those with their macrophages depleted or which were treated with anti–TNF-α antibody were able to clear the infection (Figure 7A). Despite this dichotomy in survival, intact IL-10–KO mice and those depleted of macrophages suffered the same bacterial burden (Figure 7B), reinforcing the incongruity between bacterial burden and survival.
Figure 7.
mAb-induced IL-10 works to counteract TNF-α produced by macrophages. A, IL-10–KO mice infected intravenously with 1×108 CFU Acinetobacter baumannii HUMC1 and treated with 20 μg mAb were able to survive if they were depleted of macrophages or treated with an anti–TNF-α antibody (n = 5 mice per group intact and 6 mice per group macrophage depleted); *P < .05 compared to intact IL-10–KO mice. Blood was harvested at 7 hours postinfection to quantify bacterial burden in the blood and obtain plasma cytokine levels by multiplex Luminex assay. B, Bacterial burden was no different between IL-10–KO mice with an intact immune system and with macrophages depleted; however, all intact mice died and nearly all macrophage-depleted mice survived (n = 5 mice per group). C, Cytokines showed that IL-10–KO mice depleted of macrophages had near-baseline levels of TNF-α and relatively low levels of proinflammatory IL-1β and IL-6 compared to intact IL-10–KO mice (n = 5 mice per group). B and C, Plots show medians and interquartile range. Abbreviations: CFU, colony-forming unit; IL, interleukin; KO, knockout; mAb, monoclonal antibody; Mac, macrophage depleted TNF-α, tumor necrosis factor-α.
With respect to cytokines, depleting macrophages from IL-10–KO mice resulted in near-baseline levels of TNF-α and marked reductions in proinflammatory IL-1β and IL-6 (Figure 7C). Thus, diminishing TNF-α levels by either using an anti–TNF-α antibody or depleting macrophages confers protection on treated mice, even in the absence of neutrophil-produced IL-10.
DISCUSSION
Approaches to combating antibiotic-resistant bacteria by mAb therapy have predominantly focused on evaluating efficacy via opsonophagocytosis. While the current mAb enhances phagocytosis by both murine and human macrophages, and mediates reduction of bacterial burden in vivo, we made the surprising observation that mice depleted of innate effectors died in spite of mAb-mediated reduction of bacterial burden. Reducing bacterial burden was insufficient for the antibacterial mAb to mediate clinical efficacy, as measured by survival. Mice receiving mAb therapy uniformly experienced marked reductions in bacterial density—between 2.5- and 5.5-log10-fold—regardless of whether mice were depleted of 0, 1, 2, or even 3 of their innate effectors. Despite this reduction in bacterial burden, groups depleted of neutrophils still experienced significant mortality, revealing an important mechanism by which the mAb mediates protection beyond simply reducing bacterial burden.
Rather, mAb-mediated modulation of inflammatory cytokines was necessary for protection. Subsequent experiments with knockout mice confirmed the importance of IL-10 (and not TNF-α) in mAb-mediated protection from infection. mAb-treated IL-10–KO mice died from infection, despite the bacterial burden being similarly reduced compared to wild-type controls. However, IL-10–KO mice depleted of their TNF-α–inducing macrophages or treated with an anti–TNF-α antibody were able to survive. Thus, production of TNF-α from macrophages in mAb-treated mice was antiprotective and counteracted by the protective effects of neutrophil-derived IL-10. Again, despite similar bacterial burden, macrophage-depleted and intact IL-10–KO mice experienced opposite survival outcomes and died of septic shock, as their cytokine profiles show.
In summary, the efficacy of antibacterial mAb therapy during A. baumannii bacteremia depended more on normalizing the inflammatory response to infection than on reducing bacterial burden, in line with the damage response framework of Casadevall and Pirofksi [4, 28]. Our data demonstrate that mAb therapy for one of the most antibiotic resistant gram-negative bacilli encountered in clinical medicine validates the damage response framework [9].
It is unclear how generalizable this mechanism of protection is for other mAbs against other pathogens. However, mAbs are often not evaluated with in vivo models of altered immunoregulatory phenotypes and establishment of opsonophagocytic reduction of bacterial density may have heretofore been presumed adequate evidence of that mechanism of protection. Thus, caution should be warranted in presuming mechanism of protection due to bacterial clearance, unless other immune mechanisms are also evaluated. These results may inform or alter scientific and bioengineering preclinical and clinical approaches to developing new mAb therapies for bacterial infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers R01 AI130060, R01 AI117211, R21 AI127954, and R42 AI106375 to B. S.; R01 AI139052 to B. M. L.; and R01 AI072219 to R. A. B.); and in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs Veterans Affairs Merit Review Program (grant number 1I01BX001974); and the Geriatric Research Education and Clinical Center (grant number VISN 10 to R. A. B.).
Potential conflicts of interest. T. B. N. and B. S. own equity in BioAIM LLC (Los Angeles, CA). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek 2019, Washington, DC, 4 October 2019.
References
- 1. Nielsen TB, Brass EP, Gilbert DN, Bartlett JG, Spellberg B. Sustainable discovery and development of antibiotics—is a nonprofit approach the future? N Engl J Med 2019; 381:503–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spellberg B, Nielsen TB, Gilbert DN, Shorr AF, Brass EP. Ensuring sustainability of needed antibiotics: aiming for the DART board. Ann Intern Med 2019; 171:580–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saylor C, Dadachova E, Casadevall A. Monoclonal antibody-based therapies for microbial diseases. Vaccine 2009; 27(Suppl 6):G38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nat Rev Microbiol 2004; 2:695–703. [DOI] [PubMed] [Google Scholar]
- 5. Motley MP, Banerjee K, Fries BC. Monoclonal antibody-based therapies for bacterial infections. Curr Opin Infect Dis 2019; 32:210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Babb R, Pirofski LA. Help is on the way: monoclonal antibody therapy for multi-drug resistant bacteria. Virulence 2017; 8:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buchwald UK, Pirofski L. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr Pharm Des 2003; 9:945–68. [DOI] [PubMed] [Google Scholar]
- 8. Nielsen TB, Pantapalangkoor P, Luna BM, et al. . Monoclonal antibody protects against Acinetobacter baumannii infection by enhancing bacterial clearance and evading sepsis. J Infect Dis 2017; 216:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Casadevall A, Pirofski LA. A new synthesis for antibody-mediated immunity. Nat Immunol 2011; 13:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fabrizio K, Groner A, Boes M, Pirofski LA. A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clin Vaccine Immunol 2007; 14:382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv Immunol 2006; 91:1–44. [DOI] [PubMed] [Google Scholar]
- 12. Burns T, Abadi M, Pirofski LA. Modulation of the lung inflammatory response to serotype 8 pneumococcal infection by a human immunoglobulin m monoclonal antibody to serotype 8 capsular polysaccharide. Infect Immun 2005; 73:4530–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tian H, Weber S, Thorkildson P, Kozel TR, Pirofski LA. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect Immun 2009; 77:1502–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luo G, Lin L, Ibrahim AS, et al. . Active and passive immunization protects against lethal, extreme drug resistant Acinetobacter baumannii infection. PLoS One 2012; 7:e29446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo G, Spellberg B, Gebremariam T, et al. . Diabetic murine models for Acinetobacter baumannii infection. J Antimicrob Chemother 2012; 67:1439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin L, Tan B, Pantapalangkoor P, et al. . Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 2012; 3:e00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruhn KW, Pantapalangkoor P, Nielsen T, et al. . Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J Infect Dis 2015; 211:1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nielsen TB, Bruhn KW, Pantapalangkoor P, Junus JL, Spellberg B. Cryopreservation of virulent Acinetobacter baumannii to reduce variability of in vivo studies. BMC Microbiol 2015; 15:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Talyansky Y, Nielsen TB, Yan J, et al. . Capsule carbohydrate structure determines virulence in Acinetobacter baumannii. PLoS Pathog 2021; 17:e1009291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luna BM, Yan J, Reyna Z, et al. . Natural history of Acinetobacter baumannii infection in mice. PLoS One 2019; 14:e0219824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin L, Tan B, Pantapalangkoor P, et al. . Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. MBio 2012; 3:e00312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev 2017; 30:409–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nielsen TB, Pantapalangkoor P, Yan J, et al. . Diabetes exacerbates infection via hyperinflammation by signaling through TLR4 and RAGE. mBio 2017; 8:e00818-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roghanian A, Hu G, Fraser C, et al. . Cyclophosphamide enhances cancer antibody immunotherapy in the resistant bone marrow niche by modulating macrophage FcγR expression. Cancer Immunol Res 2019; 7:1876–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bruhn KW, Dekitani K, Nielsen TB, Pantapalangkoor P, Spellberg B. Ly6G-mediated depletion of neutrophils is dependent on macrophages. Results Immunol 2016; 6:5–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Markiewski MM, Mastellos D, Tudoran R, et al. . C3a and C3b activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunol 2004; 173:747–54. [DOI] [PubMed] [Google Scholar]
- 27. Price PJ, Bánki Z, Scheideler A, et al. . Complement component C5 recruits neutrophils in the absence of C3 during respiratory infection with modified vaccinia virus ankara. J Immunol 2015; 194:1164–8. [DOI] [PubMed] [Google Scholar]
- 28. Casadevall A, Pirofski LA. The damage-response framework of microbial pathogenesis. Nat Rev Microbiol 2003; 1:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.