FIGURE 2.
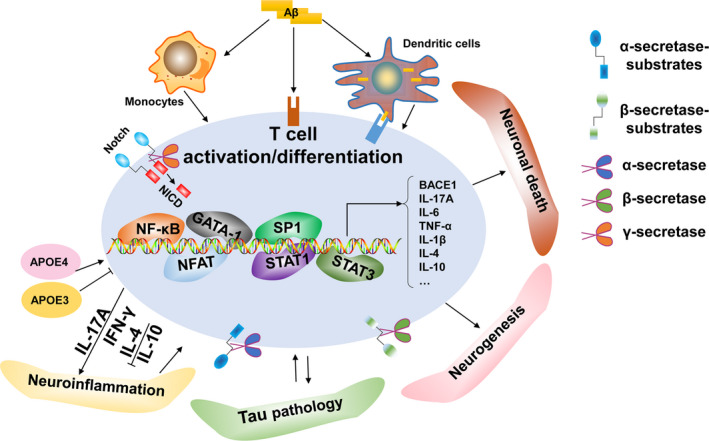
Schematic representation of the association between T cells and AD hallmarks. (1) ApoE is a key risk factor for AD and is also a significant T‐cell modulator, a function which may be ApoE‐allele‐dependent. (2) (a) Aβ can be presented by APCs to T cells as an antigen and promote T‐cell activation; (b) Aβ precursor protein endogenously expressed in T cells or exogenous Aβ directly modulates T‐cell function; (c) Aβ precursor protein expressed in monocytes induce proinflammatory cytokines to indirectly mediate T‐cell function. (3) α‐secretase mediates T‐cell function via the cleavage of diverse substrates, whereas T‐cell activation promotes α‐secretase activity. (4) T‐cell‐related biological changes regulate BACE1 expression and activity. Conversely, BACE1 may modulate T‐cell function via the cleavage of various substrates expressed on T cells. (5) The Notch receptor family are substrates of γ‐secretase, which releases the Notch intracellular domain (NICD) during proteolysis for translocation to the nucleus and activation of transcription factors involved in T‐cell development and T‐cell fate determination. (6) T cells are correlated with tau pathology and tau‐driven pathology may also induce excessive activation of T cells. (7) T cells migrate into the CNS parenchyma during the pathological progression of AD and contribute to neuronal death while T cells are also neuroprotective for spatial learning and the maintenance of neurogenesis under physiological situations. (8) T cells infiltrate the CNS and promote neuroinflammation during the pathogenesis of AD. Notably, T cells may also exhibit neuroprotective properties by regulating trophic/cytotoxic glia balance and restored glial activation
