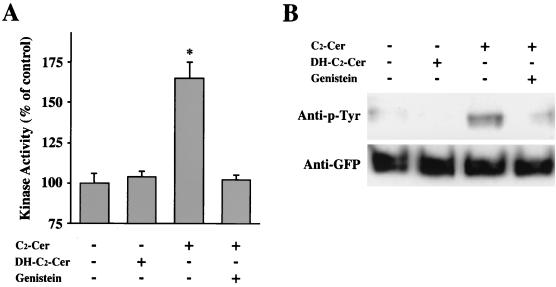
FIG. 12.
Effects of a tyrosine kinase inhibitor on kinase activity and tyrosine phosphorylation of δPKC-GFP after C2-ceramide treatment. (A) Effect of genistein on the ceramide-induced activation of δPKC-GFP assessed by in vivo kinase assay. After pretreatment with genistein (200 μM), the δPKC-GFP was immunoprecipitated from ceramide (C2-Cer)- or C2-dihydroceramide (DH-C2-Cer)-treated cells. The kinase activities of the immunoprecipitated δPKC-GFP were assayed with H1 histone as the substrate without any activators such as PS or DO. Data are expressed as percentages of the control level (the kinase activity of δPKC-GFP from untreated cells). Statistical significance: ∗, P < 0.01 versus kinase activity of control. (B) Effect of genistein on tyrosine phosphorylation of δPKC-GFP in transfected HeLa cells treated with C2-ceramide. The δPKC-GFP was prepared as described for panel A, and tyrosine phosphorylation of δPKC-GFP was analyzed by immunoblotting using anti-phosphotyrosine (anti-p-Tyr) antibody (top row). Immunoblotting of the same membrane was performed using anti-GFP polyclonal antibody as described in Materials and Methods (bottom row). All results represent the means and standard errors of more than three determinations.